Annales de Biologie Clinique
MENUComparison of two SARS-CoV-2 RT-PCR assays and implication of the instrument software on cycle threshold (Ct) value Volume 80, issue 6, November-December 2022
- Key words: Covid-19, Cycle Threshold (Ct), software, algorithm, Alinity, Abbott
- DOI : 10.1684/abc.2022.1771
- Page(s) : 537-40
- Published in: 2022
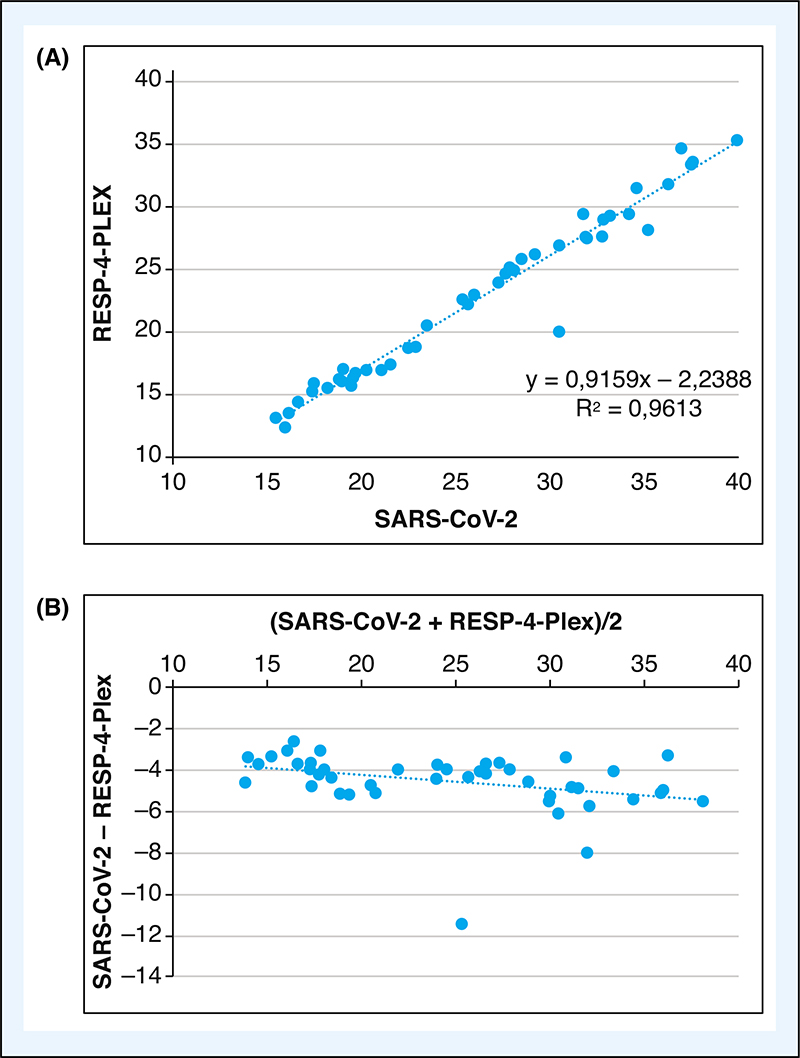
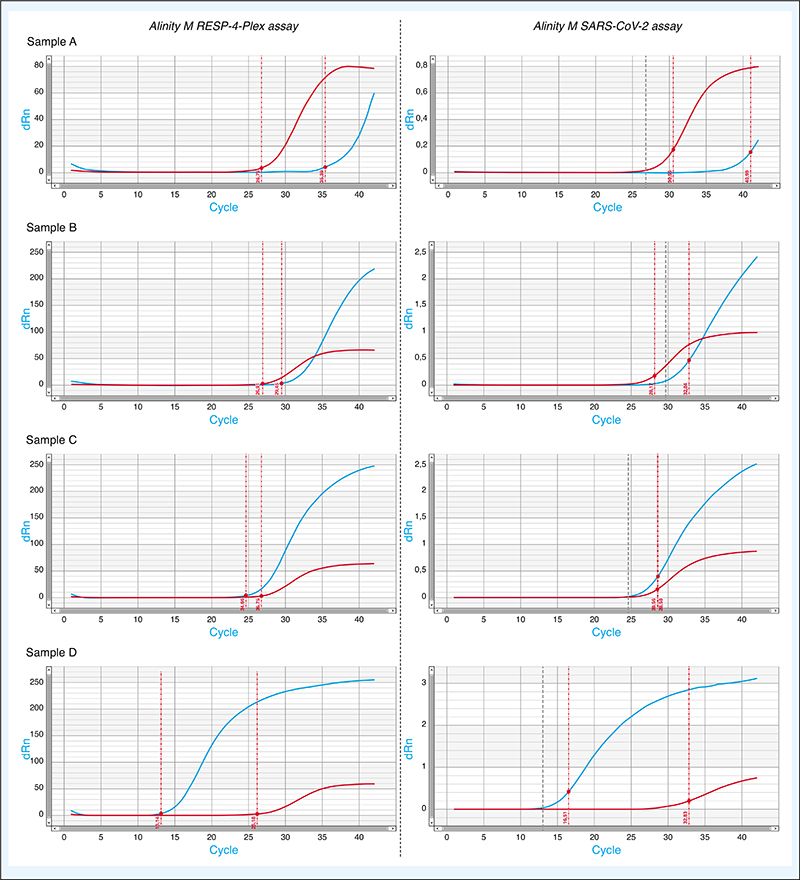
The Cycle Threshold (Ct) value of SARS-CoV-2 RT-PCR are used as an indicator of viral load. Using a collection of 45 fresh nasopharyngeal samples collected on universal transport media, we compare the Ct value obtained with 2 RT-PCR assays, the Alinity M SARS-CoV-2 and the Alinity M RESP-4-Plex (Abbott Molecular, Des Plaines, Illinois, Etats-Unis) processed on an Alinity M device. The assays are highly correlated; however, the Ct values were in median lower of 4.54 with the Alinity M RESP-4-Plex. This difference could be attributed to earlier detection of positivity by the software of the Alinity M rather than a difference in RT-PCR performances. The Ct-value of SARS-CoV-2 RT-PCR should be interpreted with caution taking into account the clinical context, pre-analytical and analytical findings.