Annales de Biologie Clinique
MENUImmuno-analytical characteristics of PSA and derived biomarkers (total PSA, free PSA, p2PSA) Volume 81, issue 1, January-February 2023
Figures
Tables
1 Laboratoire de Biochimie-Site Tenon, DMU BioGeM, AP-HP-Sorbonne Université, Département de Biochimie-Hormonologie-Suivi Thérapeutique, Hôpital Tenon, 4 rue de la Chine 75020 Paris, France
2 Sorbonne Universités, UPMC Univ Paris 6, INSERM, UMR_S 938, Centre de Recherche Saint-Antoine, Paris, France
3 INSERM, UMR_S 938, Biologie et thérapeutiques du cancer, Centre de Recherche Saint-Antoine, Paris, France
4 Institut d’Analyse Génomique, Imagenome, Labosud-Groupe INOVIE, Montpellier. France
5 Unité de Recherche Clinique. Clinique Beau Soleil, Montpellier, France
* Correspondance : C. Desbène
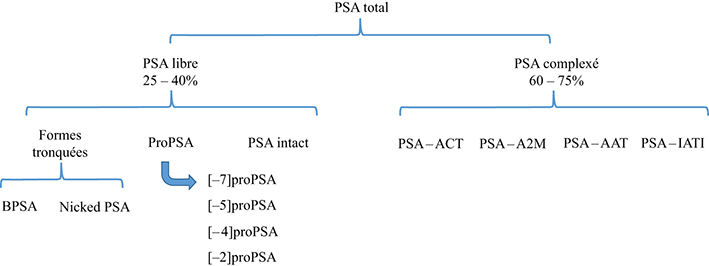
- Key words: tumor markers, prostate specific antigen, total PSA, free PSA, [-2]proPSA, prostate cancer
- DOI : 10.1684/abc.2023.1782
- Page(s) : 7-23
- Published in: 2023
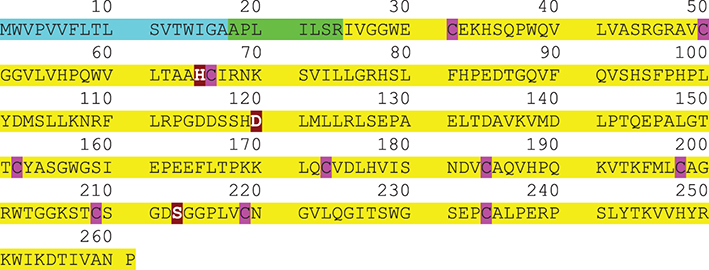
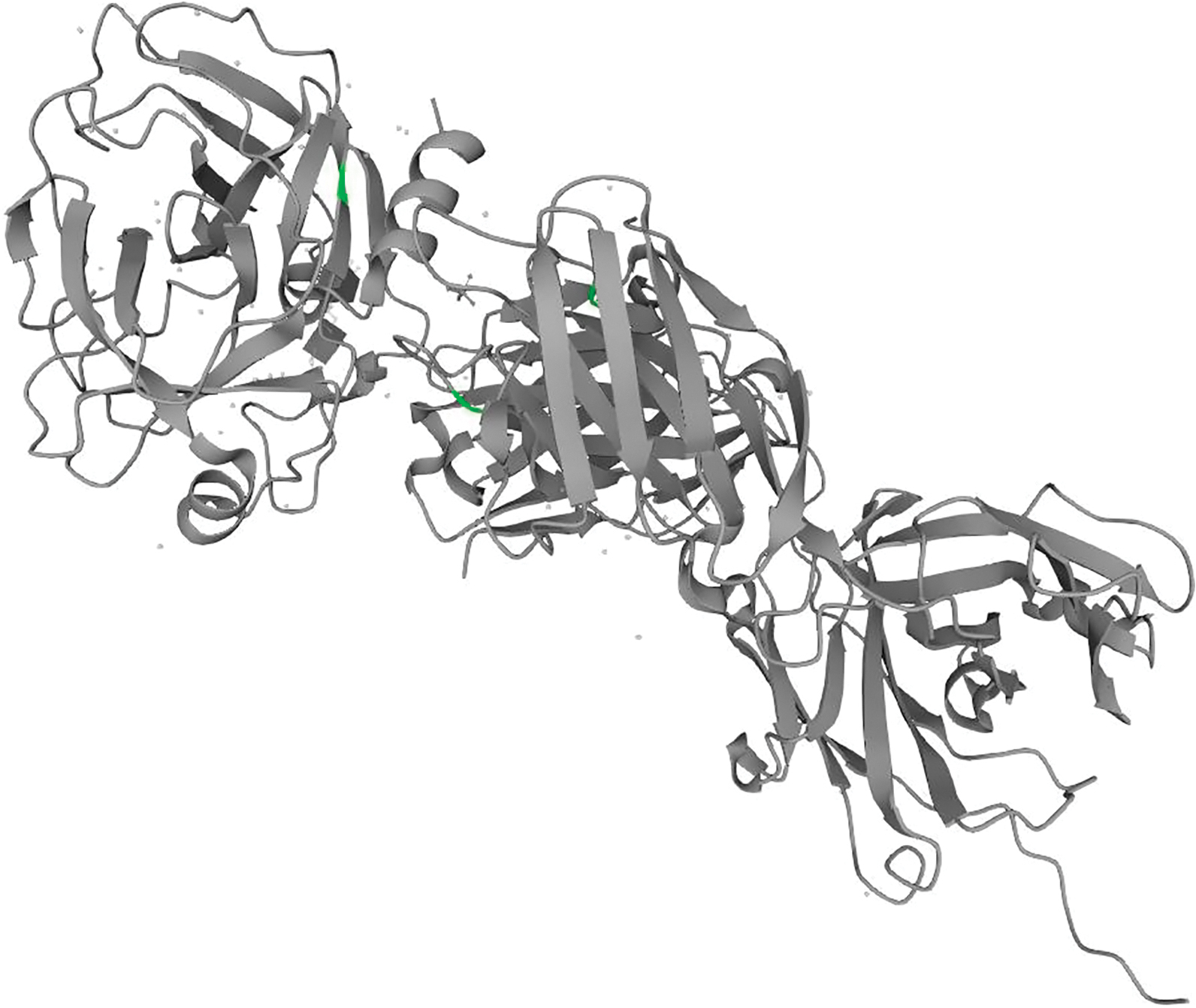
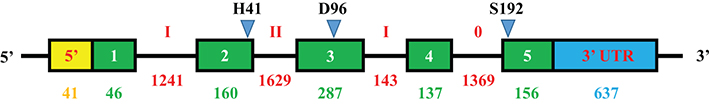
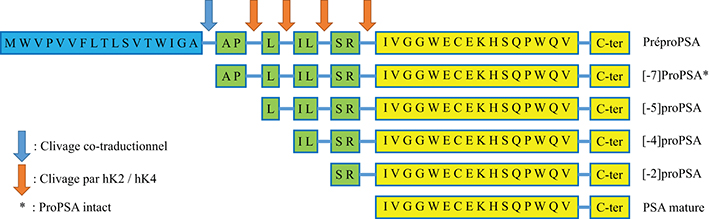
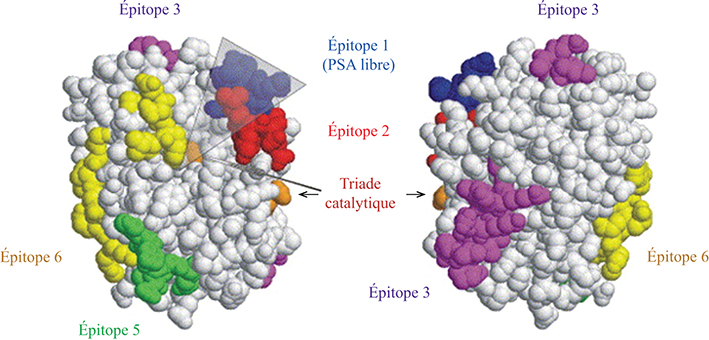
Prostate-specific antigen (PSA) is the recommended tumor marker for individual screening and follow-up of prostate cancer. This paper reviews main structural and physiological data about prostate specific antigen isoforms: total PSA, free PSA, [-2]proPSA (also named p2PSA). It describes the pre-, per- and post-analytical conditions for these different parameters. It presents the interpretation of results and derived calculated indices (free/total PSA ratio, Prostate Health Index or PHI) for the management of prostate cancer (initial diagnosis and follow-up).