Néphrologie & Thérapeutique
MENUDiagnosis and management of pruritus associated with chronic kidney disease in hemodialyzed patients Volume 20, issue 1, February 2024
- Key words: uremic pruritus, chronic kidney disease-associated pruritus, dialysis, chronic kidney disease, difelikefaline, quality of life
- DOI : 10.1684/ndt.2024.60
- Page(s) : 50-60
- Published in: 2024
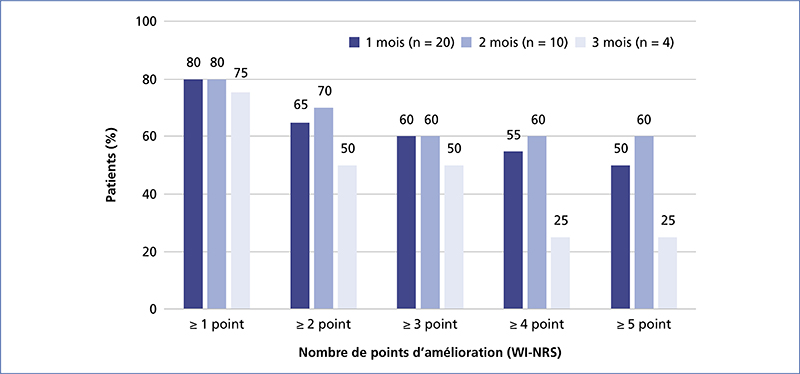
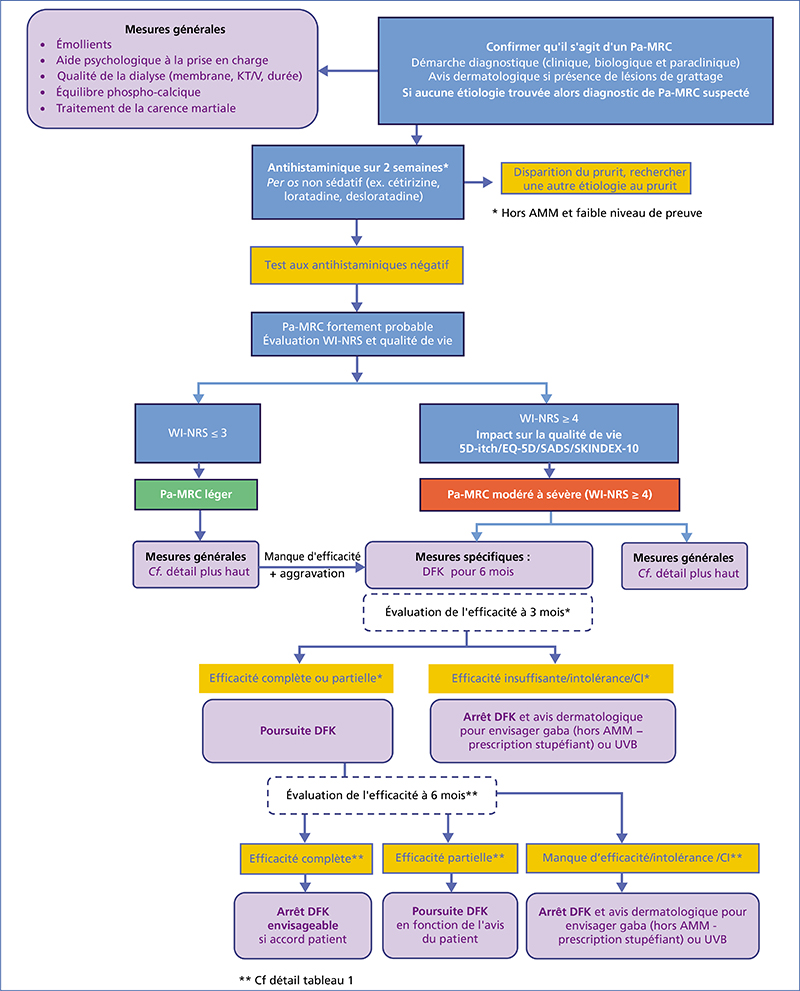
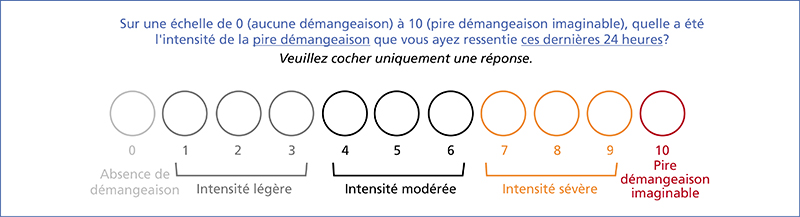
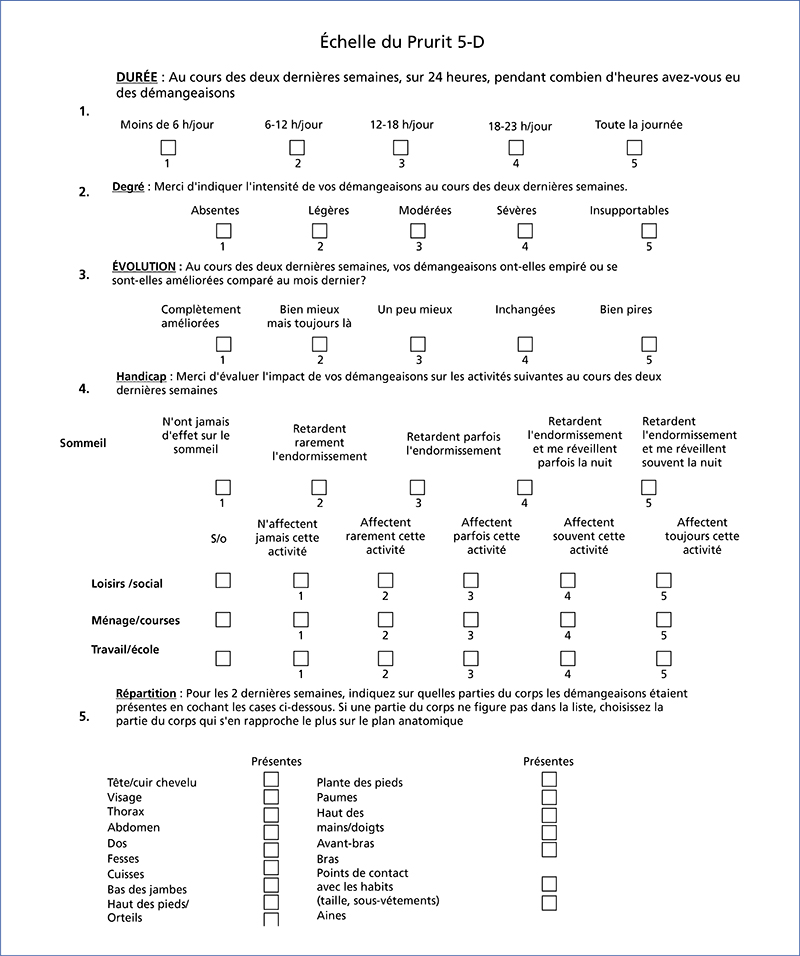
Chronic kidney disease-associated pruritus (CKD-aP) is a disabling symptom which is frequent and often underestimated. Pa-MRC has a negative impact on quality of life, and is frequently accompanied by sleep disorders and depression. The approval of difelikefalin – a kappa opioid receptor agonist – in this indication requires updated recommendations. As a first step, secondary causes of pruritus without skin lesions must be ruled out, and general measures taken (emollients, psychological support, optimization of dialysis, normalization of serum calcium, phosphate and PTH in the range proposed by the KGIDO guidelines, treatment of iron deficiency). A therapeutic test with a non-sedating oral antihistamine may be proposed. If this test is negative, Pa-MRC must be strongly suspected, and its intensity (WI-NRS scale) and impact on quality of life assessed. In the case of mild Pa-MRC (WI-NRS ≤ 3), only general measures are implemented. If Pa-MRC is moderate to severe (WI-NRS ≥ 4), specific treatment with difelikefaline can be initiated for 6 months in addition to general measures. At 3 months, if the response is complete (WI-NRS score ≤ 1) or partial (decline ≥ 3 points), treatment is continued. At 6 months, if the response is complete, treatment may be discontinued with the patient’s agreement; treatment is maintained if the response is partial. At 3 or 6 months, if response is insufficient (decline < 3 points) and/or in the event of intolerance, treatment is discontinued and an alternative treatment (e.g., gabapentinoids, UVB) may be considered after dermatological consultation.