Médecine de la Reproduction
MENUThe concerns of preimplantation genetic testing for aneuploidies: analysis and perspectives Volume 20, issue 4, Octobre-Novembre-Décembre 2018
- Key words: trophectoderm biopsy, blastocyst culture, vitrification, chromosomal mosaicism
- DOI : 10.1684/mte.2019.0725
- Page(s) : 284-98
- Published in: 2018
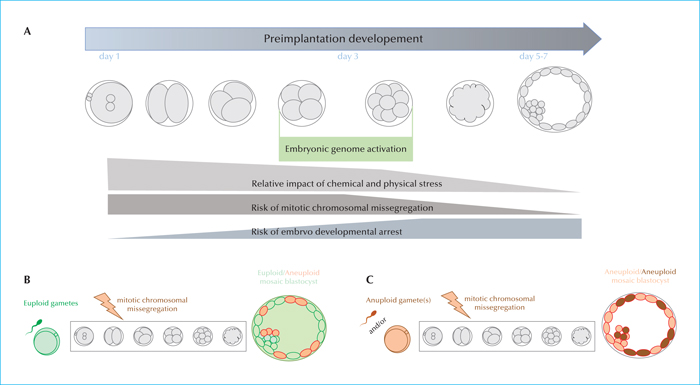
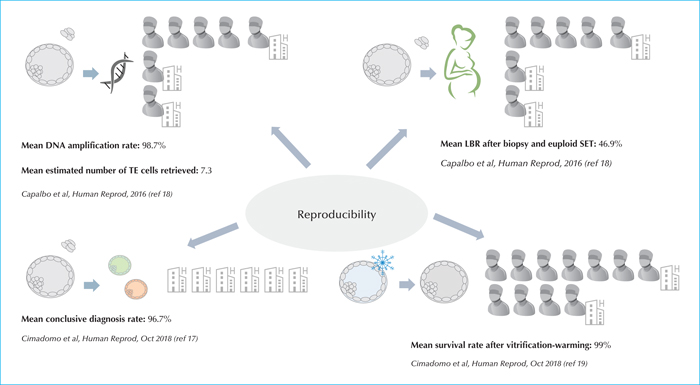
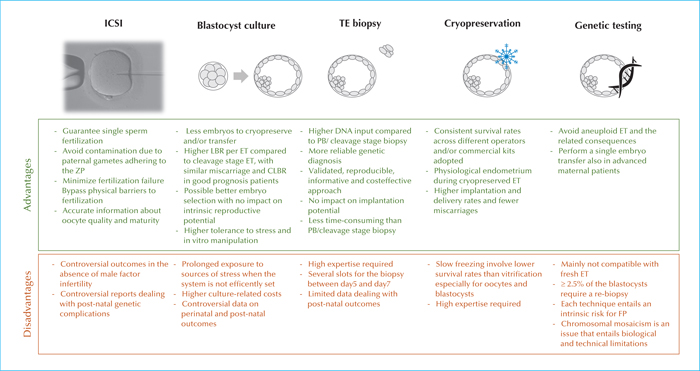
Chromosomal aneuploidies mainly affect embryos deriving from patients of advanced maternal age (AMA; >35 years), even if about 30% of the blastocysts obtained from young women might be aneuploid. To bypass the risk of transferring chromosomally-abnormal embryos (and/or embryos affected from monogenic diseases whose causative mutations were identified in the parental genotypes), pre-implantation genetic testing (PGT) has been introduced in ART. Trophectoderm biopsy is the most reliable and validated protocol to retrieve an embryonic specimen to this end. However, in order for PGT to be clinically-efficient, the IVF unit must be already proficient in ICSI, blastocyst culture and vitrification. Skilled embryologists should be familiar with all the strengths and pitfalls of each of these techniques. Similarly, the technical (e.g. manipulation-related hazards) and biological (e.g. chromosomal mosaicism) limitations of genetic testing should be clearly acknowledged from the practitioners, especially if counselling the couples indicated for PGT.
![]() This work is licensed under a
Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License
This work is licensed under a
Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License