Journal de Pharmacie Clinique
MENUStandards for the preparation of sterile products in pharmacy in Quebec Volume 38, issue 4, Décembre 2019
Figures
Tables
- Key words: standard, sterile preparations, beyond-use date, policies and procedures
- DOI : 10.1684/jpc.2019.0426
- Page(s) : 175-85
- Published in: 2019
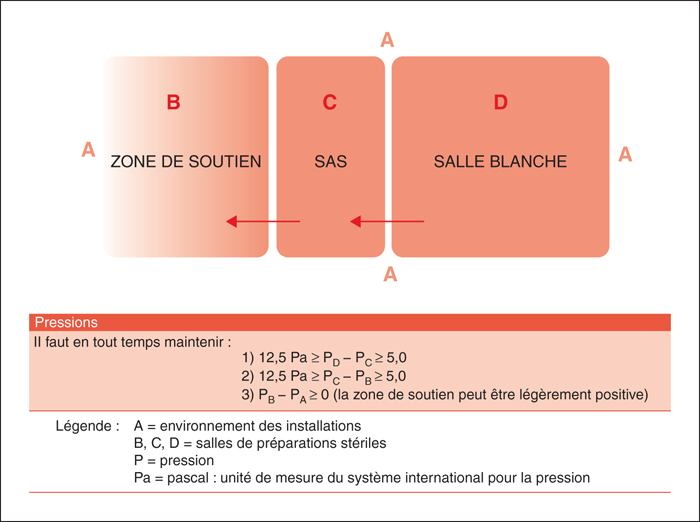
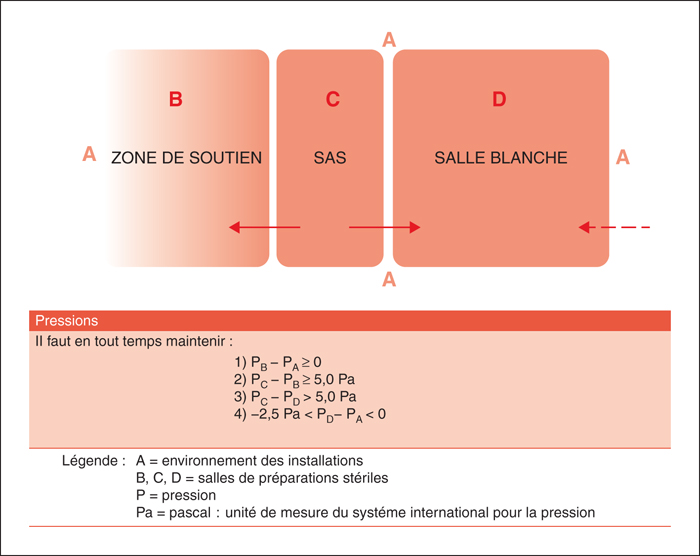
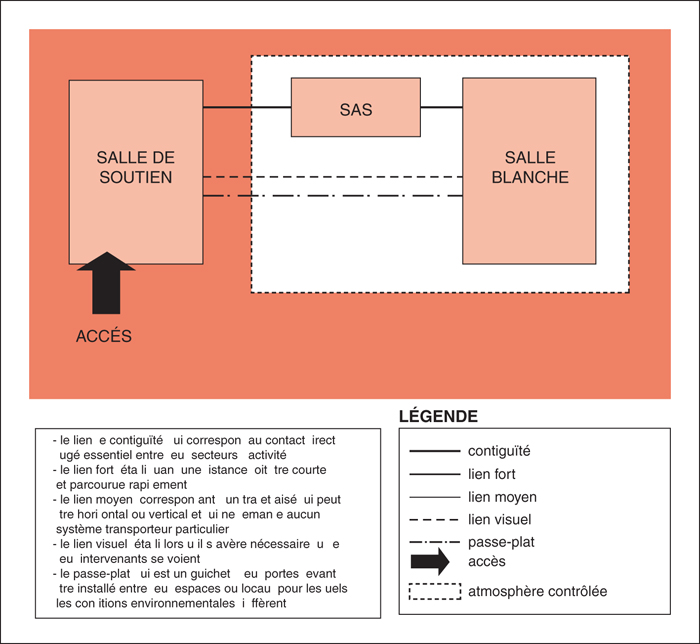
The production of sterile preparations requires a strict regulatory framework in order to standardize and optimize aseptic techniques. In spring 2014, the Ordre des pharmaciens du Québec has reviewed its existing guidelines and published two new standards for sterile preparations. Several recommendations and modifications have been added to the existing 1995 standard to provide greater public protection, including the use of the required protective garb, strict aseptic procedures and determination of the Beyond-Use Date of the preparations. Two independent standards are now available for pharmacists who supervise the production of sterile preparations in Quebec. One concerns non-hazardous sterile preparations (standard 2014.01) and the other concern hazardous sterile preparations (standard 2014.02). Pharmacist involved in this type of practice must be familiar with the various aspects of these standards in order to maintain the quality and to minimize microbial and chemical contamination. It is also important to ensure the safety of the assigned personnel to the compounding of hazardous sterile preparations which must fully respect the requirements to avoid significant risks.
![]() This work is licensed under a
Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License
This work is licensed under a
Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License