Hépato-Gastro & Oncologie Digestive
MENUAdjuvant chemotherapy for colon cancer Volume 28, issue 5, Mai 2021
Figures
- Key words: colon cancer, adjuvant chemotherapy, fluoropyrimidine, oxaliplatin
- DOI : 10.1684/hpg.2021.2180
- Page(s) : 621-6
- Published in: 2021
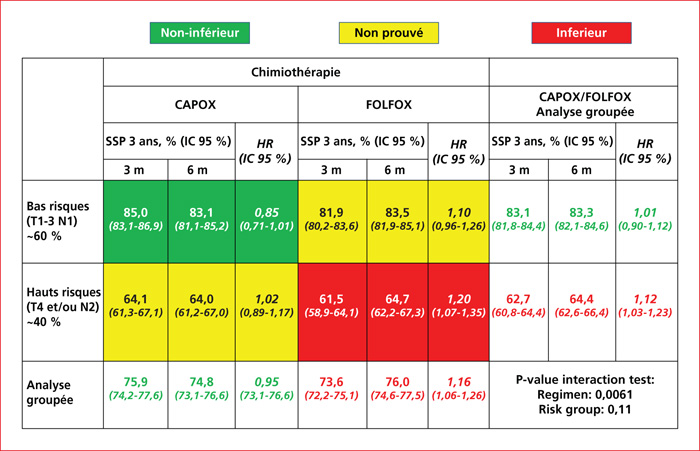
In 2021, before prescribing adjuvant chemotherapy after resection for cure for colon cancer, clinicians must consider physiological age of the patient and verify the absence of dihydroprimidine deshydrogénase deficiency. For stage II colon cancers, adjuvant chemotherapy is not considered as a standard. It could be discussed individually in a multidisciplinary concertation meeting in some situations, such as stage T4, perforation or occlusion, number of lymph nodes examined <12, poorly differentiated and/or mucoid tumor, lymphatic, venous or peri-nervous invasion. Factors contra for adjuvant chemotherapy are microsatellite instability, age and patient refusal. For low-risk stage III (T1-3 N1) tumors, chemotherapy with CAPOX (capecitabine + oxaliplatine) during 3 months should be preferred. For high-risk Stage III (T4 and/or N2): chemotherapy with FOLFOX (5-FU + oxaliplatine) during 6 months is recommended.