Hépato-Gastro & Oncologie Digestive
MENUHepatotoxicity of multikinase inhibitors Volume 28, issue 6, Juin 2021
- Key words: multikinase inhibitors, hepatotoxicity, drug-induced liver injury
- DOI : 10.1684/hpg.2021.2194
- Page(s) : 695-704
- Published in: 2021
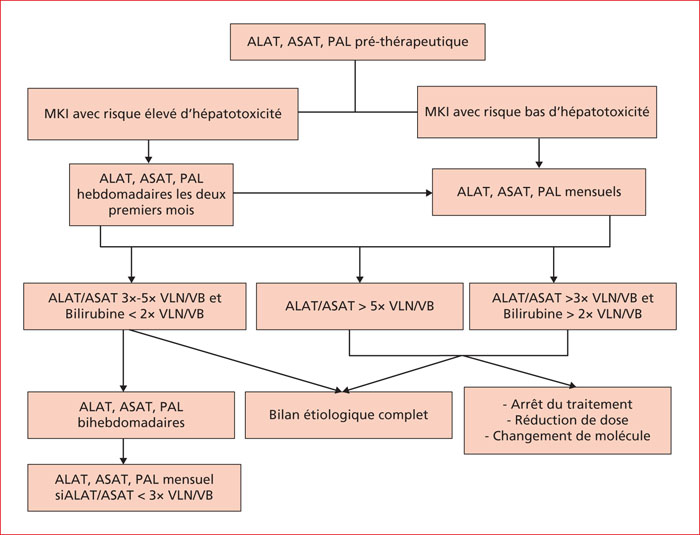
Multikinase inhibitors (MKIs) are targeted molecular agents that have revolutionized cancer management. However, data concerning MKIs-related liver injury risk and management of liver toxicity events are scarce. Asymptomatic elevation of aminotransferase levels is frequent in MKIs clinical trials, but sever hepatotoxicity is a rare instance. In most cases, latency varies between one week and two months. MKIs-related hepatotoxicity is often hepatocellular and less frequently mixed. Liver function tests spontaneously normalize following drug withdrawal in three quarters of cases, but cases of drug-induced liver injury with fatal outcome have been reported. Glucocorticoids (20-40 mg/day) were necessary in addition to drug withdrawal in case reports of hepatotoxicity related to imatinib, pazopanib, and the vemurafenib-ipilimumab combination. As there is no strategy available to prevent MKIs-related liver injury, early detection remains essential to prevent evolution to liver failure. To this end, surveillance of liver function tests during treatment may be useful. It is crucial that potential causes of hepatic injury be excluded to avoid unnecessary withdrawal of these molecules.