Hépato-Gastro & Oncologie Digestive
MENUTreatment of advanced squamous-cell anal carcinoma Volume 27, issue 9, Novembre 2020
- Key words: squamous-cell anal carcinoma, metastatic, advanced, chemotherapy, treatment
- DOI : 10.1684/hpg.2020.2025
- Page(s) : 873-81
- Published in: 2020
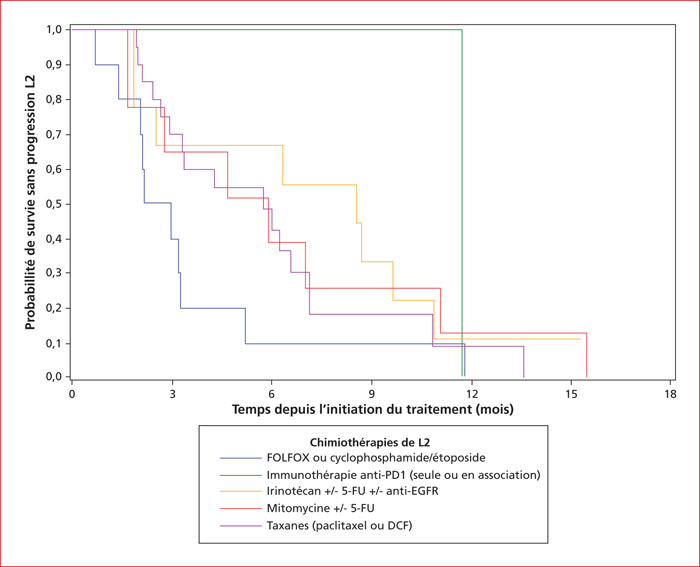
Anal canal carcinoma represents 2.7% of gastrointestinal cancers, and its incidence is steadily increasing. Squamous cell carcinoma is by far the most frequent subtype, and is associated with HPV infection, mostly the genotype 16 in 90% of cases. Approximately 30 to 40% of patients are in an advanced stage, either with metastatic disease at diagnosis or with non resectable recurrence after chemoradiotherapy for an initially localized disease. Systemic chemotherapy is then the standard of choice. In France, a national network was settled and made possible to perform large phase II trials in advanced anal carcinomas. This is a paradigm shift with prospective validation of new strategies in this rare clinical setting thanks to the collaboration of different groups. Its first fruit was the Epitopes-HPV02 trial which validated the modified DCF (docetaxel, cisplatin, and 5-fluorouracil) regimen as a new standard. More recently, an international network has also validated the interest of taxanes in first-line. In second-line, results with anti-PD1/PD-L1 check point inhibitors are encouraging, especially in association, and could be the next standard. Several clinical trials are ongoing