Hépato-Gastro & Oncologie Digestive
MENUSide effects of long-term Inflammatory Bowel Disease treatments Volume 28, issue 1, Janvier 2021
- Key words: side effects, inflammatory bowel disease, biotherapy, immunosuppressant, 5-aminosalicylates
- DOI : 10.1684/hpg.2020.2099
- Page(s) : 54-66
- Published in: 2021
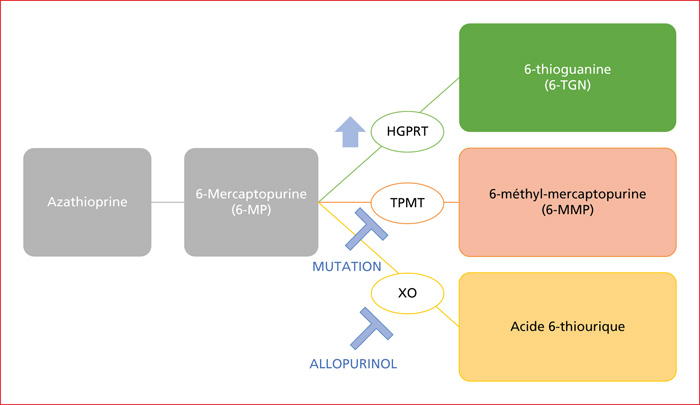
The course of chronic inflammatory bowel disease (IBD) has been significantly altered by the development of substantive therapies, including immunosuppressants and biotherapies. Their prolonged use, however, exposes the patient to many long-term side effects that are partially known. Aminosalicyl derivatives have renal toxicity due to an interstitial nephropathy, which should be detected by regular biological monitoring (incidence of about 0.2 %). The main risks of thiopurines are infectious, neoplastic (cutaneous, urinary, gynaecological and haematological) and hepatic (port-sinoidal vascular disease). Methotrexate exposes to the risk of hepatic and pulmonary fibrosis. This molecule is also teratogenic and its hematotoxicity is prevented by the administration of folate. Anti-TNF drugs expose to long-term infectious and oncological risks. They require monitoring of the vaccination schedule, screening before the initiation of this treatment and regular screening during the course of treatment. More recently, dugs as vedolizumab and ustekinumab appear to have a better safety profile but we lack of long-term follow-up studies. Apart from the risk of shingles, the main adverse events reported with JAK inhibitors are dyslipidemia and thromboembolic events. Once again, we lack hindsight to conclude. The long-term side effects of IBD treatments require compliance with their main contraindications, precautions in terms of infection and targeted and regular screening for possible neoplastic lesions.