Epileptic Disorders
MENUILAE Neuroimaging Task Force Highlight: harnessing optimized imaging protocols for drug-resistant childhood epilepsy
Volume 23, issue 5, October 2021
Case presentation
A 13-year-old, right-handed female presented with drug-resistant focal epilepsy. Her first seizure, at the age of seven years, was characterized by a headache, colored spots across her entire visual field, and loss of consciousness, eventually progressing to eyes rolling up and foaming from her mouth. From age 13 years onwards, her second and subsequent seizures began with initial symptoms of a “wave of emotion”, an epigastric sensation, and a sensation of chest tightness followed by a feeling of dissociation that she described akin to being “in a bubble.” Her seizures typically lasted seconds to minutes and evolved to aphasia, a fearful or blank facial expression, an inability to move, hypersalivation/spitting, right side dysesthesia, stereognosis, and rightward head version.
After her first seizure, carbamazepine was started but was discontinued due to rash and fever. She remained off medication until her second seizure at age 13 years. For nearly two years, she was maintained on levetiracetam and remained completely seizure-free. Her seizures returned after levetiracetam was weaned off. Over the following year, her seizure frequency increased up to 20/day, and she suffered an episode of non-convulsive status epilepticus, lasting up to 30 minutes. She was treated with 1,000 mg and 1,500 mg levetiracetam (50 mg/kg/day), 100 mg lacosamide (4 mg/kg/day), 30 mg clobazam (0.6 mg/kg/day), and rescue midazolam. She previously tried lamotrigine, which was discontinued due to drug rash with eosinophilia and systemic symptoms syndrome.
Her birth and developmental history were normal, and she had no epilepsy risk factors (e.g., no febrile seizures) aside from a fall from her highchair when she was 2.5 years of age. Her younger brother had a simple febrile seizure. She also had an uncle who presented with seizures at age nine years, which resolved in adulthood.
Presurgical investigation and postsurgical outcome
Electroclinical and neuropsychological assessment
Initial investigations at our center, at age 13, included a routine EEG that captured a 10-second subtle electrographic seizure over the left anterior temporal regions (maximal at F7). Before starting the levetiracetam taper, she completed a 24-hour ambulatory EEG and 72-hour inpatient video-EEG monitoring, which did not capture clinical events. When she was completely off levetiracetam, and her seizures had returned, a repeat four-day inpatient video-EEG recorded 10 seizures characterized by behavioral arrest with variable preservation of memory that were at times preceded by eye movements and gagging. Ictal EEG showed a focal evolving theta rhythm over the left frontotemporal regions. Her interictal EEG showed frequent left frontotemporal spikes mixed with intermittent slowing. No interictal abnormalities were recorded from the occipital leads.
Her presurgical cognitive abilities were assessed using the WISC-V Canadian (Wechsler Intelligence Scale for Children-Fifth Edition: Canadian) [1], D-KEFS (Delis-Kaplan Executive Function System) Verbal Fluency [2], and WRAML2 (Wide Range Assessment of Memory and Learning Second Edition) Story Memory [3]. Her performance across cognitive domains was largely within to above the range expected for age (supplementary table 1). Verbal comprehension scores were with average range. Working memory abilities were found to vary in the average to low-average range. Verbal fluency was in the average to high-average range, as were inhibition and cognitive switching abilities. Her performance during memory testing varied from average (visual memory) to high-average (verbal memory) range. Functional MRI lateralized language to the left hemisphere. Given her high level of function and reluctance to undergo surgery, CSF testing for autoantibodies was performed to exclude potentially treatable causes. These tests were unremarkable. Similarly, targeted whole-exome sequencing revealed no pathogenic mutations attributable to her epilepsy.
Clinical and research MRI investigation
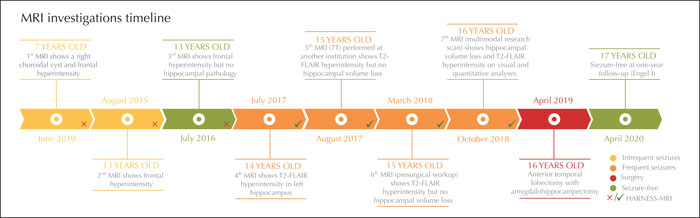
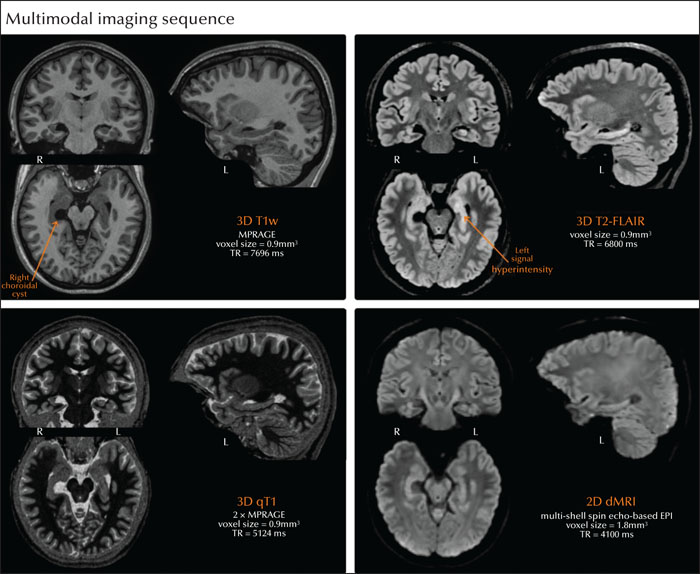
The patient underwent extensive MRI investigations in several centers; the first three MRIs, however, did not follow optimized epilepsy protocols (figure 1) [4]. Her first two MRIs were performed at ages seven and 13 in a different country and revealed non-specific foci of mild T2 hyperintensity in the anterior frontal lobes and a benign right choroidal fissure cyst. Her third MRI, at age 13, was performed upon admission at our center; visual MRI analysis also revealed frontal white matter changes but no hippocampal pathology. Subtle mesiotemporal lobe abnormalities in these three initial MRI scans were not identified based on retrospective visual analysis by two authors (SL and BCB). A fourth MRI, at age 14 -a time of frequent seizures-, showed increased T2-FLAIR signal intensity in the left hippocampus proper. A month later, and still hesitant to undergo surgery, the patient sought a second opinion and underwent a 7T MRI at another institution; findings confirmed persistent, albeit improved, T2-FLAIR signal hyperintensity in the left hippocampus but no hippocampal volume loss. Similar findings were reported following her sixth MRI scan, which was acquired during her presurgical workup. Six months prior to surgery, at age 16 years old, the patient underwent a multimodal research 3T MRI scan with prospective motion correction, including a (i) 3D T1-weighted anatomical scan, (ii) 3D T2-FLAIR, (iii) 3D quantitative T1 mapping, and (iv) 2D diffusion MRI. Real-time prospective motion correction [5] reduces head motion and distortion artifacts in high-resolution 3D MRI scans, and may therefore facilitate identification of subtle pathological findings in pediatric and clinical populations. In line with the HARNESS-MRI protocol (harmonized neuroimaging of epilepsy structural sequences), the 3D scans had isotropic voxel sizes of 1 mm or less [4]; details on the MRI acquisition can be found in the supplementary material.
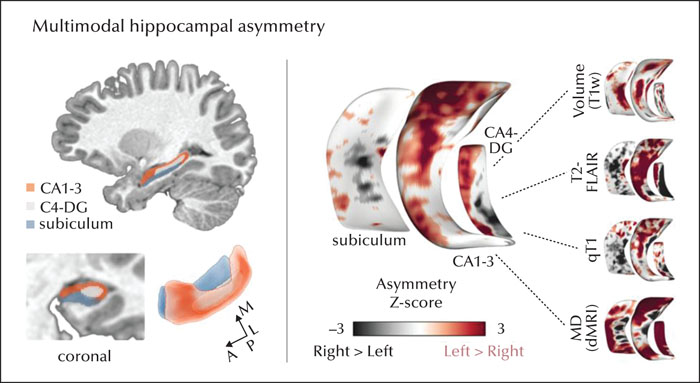
Visual inspection of the MRI scans shows a right choroidal fissure cyst and conspicuous T2-FLAIR signal hyperintensity in the left, relative to the right, hippocampus (figure 2). Quantitative analyses of these multimodal imaging data in the patient, standardized relative to data from eight healthy controls [6, 7], localized marked changes (i.e., increased atrophy, increased mean diffusivity, increased T2-FLAIR signal, and lower qT1 values) in the anterior portions of CA1-3 and CA4-DG (up to z=9.30) in the left, relative to right, hippocampus (figure 3).
Surgical intervention and postsurgical findings
The patient underwent a left anterior temporal lobectomy with amygdalohippocampectomy at age 16 years. Histopathology of the resected specimen revealed hippocampal sclerosis with widespread gliosis of the hippocampus, conspicuous focal neuronal loss in CA1, and some focal neuronal loss in the dentate granule layer of the dentate gyrus. Gliosis was also observed in the amygdala and lateral temporal lobe, albeit mild. At a follow-up visit at 18 months, she has remained seizure-free since surgery (ILAE 1).
A follow-up neuropsychological evaluation performed 18 months after surgery revealed that her overall cognitive skills continue to be largely within to above the range expected for age. Although administered using slightly different measures pre- (WISC-V Canadian [1], D-KEFS Verbal Fluency [2], WRAML2 Story Memory [3]) and postoperatively (WAIS-IV, Wechsler Adult Intelligence Scale Fourth Edition [10]; D-KEFS [2]; CPT-III, Continuous Performance Test Third edition [11]), only mild changes in language and aspects of verbal memory were observed, moving from the high-average (prior to surgery) to the low-average (after surgery) range when compared to age-matched peers (supplementary table 2). While other cognitive skills remain strong, language and verbal memory changes may have been, in part, expected given the site of surgery (left anterior temporal lobe).
Discussion
This case highlights the need for optimized data acquisition epilepsy protocols early in the clinical course, ideally at onset of diagnosis. While the patient's first three MRIs revealed a benign right choroidal fissure cyst, together with non-specific frontal lobe signal hyperintensity, mesiotemporal anomalies may have been initially overlooked. This could be due to a number of reasons, including the use of non-HARNESS-MRI scan protocols (2D sequences, low resolution) and the absence of quantitative volumetry analysis. On the other hand, follow-up MRI scans throughout the patient's course of treatment, particularly at a time of high seizure frequency, revealed evidence of mesiotemporal signal hyperintensity and may, to some extent, reflect acute changes related to an increase in seizure frequency [12-14]. Notably, these MRI scans (from the fourth scan onwards) followed HARNESS-MRI guidelines established by the ILAE [4], specifically incorporating high-contrast 3D sequences with isotropic millimeter voxels, consequently solidifying the diagnosis of hippocampal sclerosis and thus supporting surgical candidacy. Although we cannot disregard the hypothesis that mesiotemporal lobe pathology was initially non-existent and stemmed from an increase in seizure frequency, recent work in patients with newly diagnosed focal epilepsy suggests that hippocampal atrophy is already present at disease onset and may not be a consequence of the chronicity of the disorder, nor of other clinical characteristics [15]. The use of optimized protocols from the outset could have provided earlier neuroradiological evidence of hippocampal sclerosis in the clinical course, which may have led to streamlined referral for epilepsy surgery and treatment, reducing the potential risk of injury and seizure-related side effects. Moreover, individuals who benefit from rapid surgical interventions, and consequently have shorter epilepsy durations, are more likely to achieve seizure freedom at follow-up [16].
Alongside the demonstrated use of optimized epilepsy neuroimaging protocols as well as the provision of clinically relevant information when reviewing MR images [17,18], a large body of literature supports quantitative analysis of MRI contrasts to enhance personalized diagnosis and prognosis of drug-resistant patients with epilepsy [6, 9, 19-21]. Established imaging markers of epilepsy-related hippocampal pathology include hippocampal morphology seen on T1-weighted images and an increased T2-weighted signal, two features sensitive to hippocampal cell loss and reactive astrogliosis, respectively [22-24]. In this patient, quantitative analysis of these features along hippocampal subfield surfaces, together with diffusion and quantitative T1 MRI features, indicated left-lateralized mesiotemporal anomalies. Although this analysis was confirmatory in the current patient, as hippocampal anomalies were already apparent based on visual inspection, the use of multimodal postprocessing methods has shown promise in identifying subtle mesiotemporal pathology that may be initially overlooked by conventional neuroradiological examination. Close coordination among epileptologists, radiologists, and epilepsy research scientists can facilitate the integration of advanced neuroimaging techniques into the preoperative clinical workup. Aside from assisting in surgical target definition, longitudinal quantitative imaging analyses throughout treatment could distinguish pre-existing abnormalities from those progressing from (i) an initial precipitating insult, (ii) an episode of status epilepticus, or (iii) a high seizure burden. Therefore, high-quality and multimodal MRI acquisition and quantitative postprocessing methods may have practical implications for rapid and precise diagnosis as well as early referral for presurgical investigations in children and adults with drug-resistant focal epilepsy.
Supplementary material
Supplementary material and summary slides accompanying the manuscript are available at www.epilepticdisorders.com.
Disclosures
The authors did not declare their potentiel conflicts of interest.
![]() This work is licensed under a
Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License
This work is licensed under a
Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License