Epileptic Disorders
MENUFacing the hidden wall in mesial extratemporal lobe epilepsy Volume 20, issue 1, February 2018
Effectiveness and superiority of surgical treatment has been demonstrated for drug-resistant temporal lobe epilepsy (TLE). Extratemporal lobe epilepsy (ETLE) makes up a heterogeneous entity but is approached with much greater care since the outcome tends to be inferior to temporal lobe epilepsy surgery (Cascino et al., 1992; Tellez-Zenteno et al., 2005; Spencer and Huh, 2008). However, while the number of mesial TLE surgeries is declining, the proportion of MRI-negative and ETLE surgery has increased during the last decade (Jehi et al., 2015).
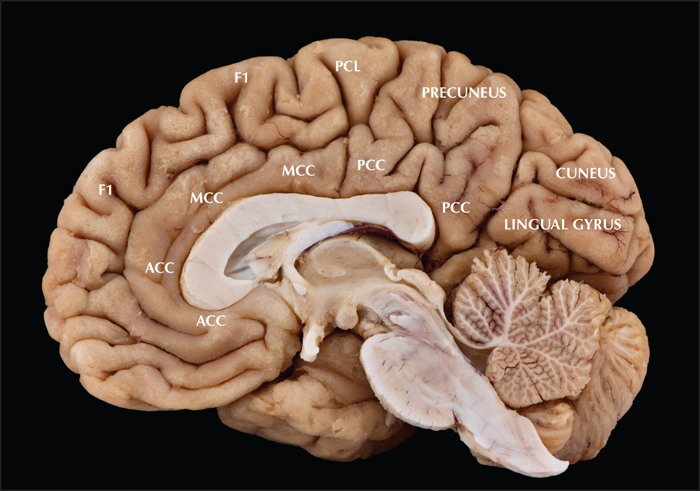
Most reports on ETLE describe their results according to the presumed lobe of origin whether frontal, parietal, occipital or insular (Blume et al., 1991; Salanova et al., 1992; Williamson et al., 1992a; Williamson et al., 1992b; Salanova et al., 1995; Aykut-Bingol et al., 1998; Jobst et al., 2000; Binder et al., 2008; Binder et al., 2009; Von Lehe et al., 2009; Englot et al., 2012). Few studies only focus specifically on epilepsy surgery performed in mesial extratemporal areas (So, 1998; Blume et al., 2005; Leung et al., 2008; Kasasbeh et al., 2012; Unnwongse et al., 2012; von Lehe et al., 2012; Alkawadri et al., 2013; Theys et al., 2017). Epileptogenic zones encompassing the cortex of the mesial wall of the brain pose several difficulties since this hidden cortex is difficult to access, both diagnostically as well as therapeutically. The epileptogenic cortex can also overlap with a wide variety of functional areas including primary cortical areas (visual [V1], somatosensory [S1], and motor [M1]), the supplementary motor area (SMA), prefrontal areas, cingulate areas, and the precuneus (figure 1). Although mesial ETLE cases involve functionally heterogeneous areas, they share common diagnostic and surgical principles. Besides classic electroclinical diagnostics, defining the epileptogenic zone (EZ) often involves advanced multimodal imaging combined with intracranial EEG recordings, even more so for MRI-negative cases.
In this review, we focus specifically on mesial ETLE surgery and the different strategies to cope with the inherent diagnostic and surgical difficulties. After good diagnostic workup, satisfactory surgical results can be obtained in this difficult patient group. Defining the epileptogenic cortex and tailoring surgical resection is often based on targeted intra- or extra-operative intracranial recordings.
Diagnostic approach
Concordance of electroclinical findings: the exception rather than the rule
Routine clinical workup with a detailed history and the use of video-EEG remains indispensable in the assessment of ETLE, but congruent findings are certainly not the rule in ETLE (Remi et al., 2011). The epileptogenic cortex can either be symptomatogenic or silent, and although the localizing value of semiology can be low, semiological findings possess an important, but not absolute, lateralizing value (Boesebeck et al., 2002).
The functional diversity of the mesial cortex is complex, and the knowledge of these brain regions such as the cingulate has expanded rapidly (Vogt, 2005), however, there is a marked lag in terms of clinical epileptology. The diversity of ictal signs in mesial frontal lobe seizures reflects the complex long and short range connectivity of this region, since seizure semiology tends to reflect seizure spread. An urge to move and ictal body turning around the horizontal body axis have been specifically associated with mesial frontal epilepsy (Leung et al., 2008). Frontal hypermotor seizures (HMS) can have different presentations according to the location of the epileptogenic zone (EZ) along an antero-posterior gradient; while an EZ in the ventromesial prefrontal cortex is associated with fearful agitation, a location in the mesial premotor cortex can be associated with horizontal and rotational body movements (Rheims et al., 2008; Bonini et al., 2014). Supplementary motor area (SMA) seizures (Morris et al., 1988), preceded by a (mostly somatosensory) aura in half of the cases typically present with asymmetric tonic posturing. Other frontomesial seizure types include so-called frontal absence seizures, which can be co-existent with different seizure types (Bancaud et al., 1974; So, 1998; Chassagnon et al., 2009). Versive seizures and atonic seizures are less frequent manifestations of frontomesial epilepsy. Anterior cingulate cortex (ACC) epilepsy also typically presents with frontomesial ictal semiology, such as hypermotor seizures, tonic posturing, and early loud vocalization (von Lehe et al., 2012; Alkawadri et al., 2013). A clinical sign typically ascribed to initial ACC involvement is the “chapeau de gendarme”, a commonly used French epileptological term, typically regarded as a tonic bilateral contraction of the mouth with the corners of the lip down-turned (Souirti et al., 2014). The English term “ictal pouting”, however, does not describe this mouth position and rather refers to pursing of the lips as if to blow a kiss. Laughter and mirth have also been associated with ACC and frontomesial seizures (Chassagnon et al., 2003; Unnwongse et al., 2010; Caruana et al., 2015;).
Posterior cingulate cortex (PCC) epilepsies can present as simple tonic or hypermotor seizures with typical auras being vestibular or dyscognitive (Alkawadri et al., 2013; Montavont et al., 2013; Enatsu et al., 2014). Central lobe epilepsy is associated with focal somatosensory manifestations or with clonic seizures and epilepsia partialis continua (EPC) (Chauvel et al., 1992; Tuxhorn, 2005). A somatosensory aura or a sensation of vertigo can direct towards a parietal lobe onset. Mesial parietal epilepsy is more often associated with automotor seizures than frontomesial epilepsy, but can also present with tonic or versive seizures (Bartolomei et al., 2011; Ristic et al., 2012). Manifestations in occipital lobe epilepsy can include negative or positive visual symptoms, blinking, and versive movements (Williamson et al., 1992b; Salanova et al., 1992; Jobst et al., 2010). Mesial occipital epilepsy is associated with visual hallucinations in 60-75% of cases (Boesebeck et al., 2002; Blume et al., 2005).
In cases where the ictal onset zone is clinically silent, seizures can rapidly spread and semiology will represent propagated activity. PCC epilepsy can often present as temporal lobe epilepsy (Koubeissi et al., 2009; Alkawadri et al., 2013). Other mesial ETLE, in particular posterior cortex epilepsies, can also present as pseudotemporal epilepsy (Jehi et al., 2009).
Not only can clinical symptoms result from propagation, scalp EEG often reveals propagated activity. Temporal EEG abnormalities are frequently found and can be misleading, even more so when the interhemispheric discharges are not picked up. In parasagittal epilepsies, the EEG can often lateralize but does not usually allow for localization. In frontal lobe epilepsy, rhythmic midline theta activity can be found (Beleza et al., 2009), as well as midline spikes, and secondary bilateral synchrony and abnormalities are present in the majority of cases (Salanova et al., 1995; Wieser and Hajek, 1995; Boesebeck et al., 2002). The EEG can thereby mimic multifocal or generalized epilepsy, which could inadvertently exclude patients that would benefit from resective surgery.
Overall, electroclinical findings have a relatively good lateralizing value but tend to have a poor localizing value, making the diagnosis of mesial ETLE challenging (Tukel and Jasper, 1952).
Multimodal imaging and advanced imaging
Since MRI-positive epilepsy is clearly associated with a better outcome (Chapman et al., 2005; Bien et al., 2009; Noe et al., 2013), it is of paramount importance not to overlook discrete cortical signal alterations. Indeed, small bottom-of-sulcus dysplasias or slight grey-white matter alterations can be easily overlooked on routine imaging. MRI postprocessing (Wang and Alexopoulos, 2016) and advanced imaging techniques can sometimes convert MRI-negative into MRI-positive cases and multimodal imaging can also optimize or confirm the working hypothesis (Knowlton et al., 2008).
The role of interictal positron emission tomography (PET), mainly using 18F-FDG, in mesial epilepsy has not been studied. In TLE, there is no clear added value of FDG-PET in localizing the epileptogenic zone (Willmann et al., 2007). However several studies in TLE have shown that when focal FDG-PET hypometabolism is present in patients with normal MRI, surgical outcome is equivalent to those with clear MRI lesions (Carne et al., 2004; Lopinto-Khoury et al., 2012; Gok et al., 2013; Yang et al., 2014). PET seems more predictive in patients with TLE than in those with ETLE (Rathore et al., 2014).
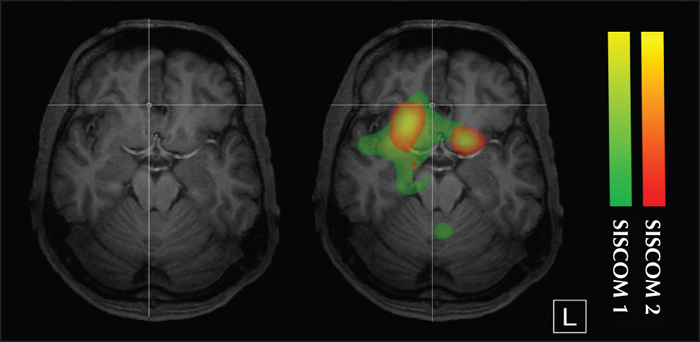
Subtracted ictal SPECT co-registered to MRI (SISCOM) is a multimodal image which combines structural information, including the epileptic lesion, with ictal perfusion changes (Van Paesschen et al., 2007; Goffin et al., 2008). Results are heavily dependent on the timing of the tracer injection; when performed late it can be false localizing or non-contributory. Easy access to nuclear imaging is essential for implementation of this modality in the routine work-up of epilepsy surgery candidates. It remains the only imaging modality which is used to visualize the ictal onset zone on a routine basis. Since focal dysplastic lesions are intrinsically epileptic, hyperperfusion typically overlaps with these lesions (Dupont et al., 2006). The combination of SISCOM with morphometric analysis of the MR images (Wellmer et al., 2010; Wagner et al., 2011; House et al., 2015) is a powerful tool in the presurgical evaluation of patients with subtle cortical dysplastic lesions, often making it possible to delineate the epileptogenic zone non-invasively (figure 2). Epileptic lesions other than dysplastic lesions show ictal hyperperfusion immediately surrounding the epileptic lesion (figure 3). SISCOM can delineate the ictal onset zone in mesial extratemporal lobe epilepsy in a non-invasive manner (figure 2 and 3).
After imaging has demonstrated an epileptogenic lesion, intraoperative electrocorticography (ECOG) may guide surgical resection, an approach which has been shown to be successful for focal cortical dysplasia (FCD) (Harvey et al., 2015).
The case for magnetoencephalography
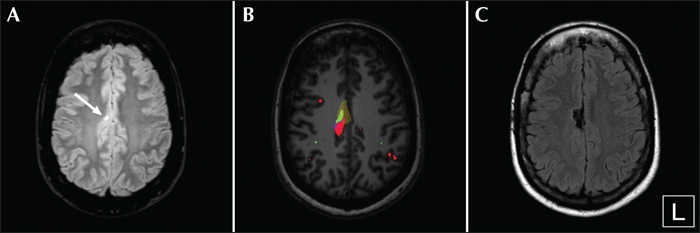
Magnetoencephalography (MEG) is a non-invasive neurophysiological technique that is highly sensitive to cortical sources that are tangential to the skull and, in comparison with EEG, is almost blind to radial sources. The heightened sensitivity of MEG to fissural/tangential cortical sources, together with some differences in cortical signal to noise ratio (SNR) (Goldenholz et al., 2009), explains why MEG can detect epileptic activity which is not captured by EEG (and vice versa) (see e.g. Iwasaki et al. 2005 and Knake et al. 2006). When combined with structural MRI, MEG signals can be used to estimate the location of epileptic sources with a spatial resolution of a few millimetres (i.e. magnetic source imaging [MSI]). In epileptic patients, MEG investigations mainly provide information about the irritative zone as ictal recordings are seldom performed due to practical constraints (e.g. difficulty to maintain the patient in the magnetic shielded room for a prolonged time) (Medvedovsky et al., 2012; Badier et al., 2016). In the context of mesial ETLE, the capability of MEG to detect neural activity located in the mesial wall of the brain remains a matter of debate and crucially depends on SNR issues (e.g. level of noise in the data, extension of the activated cortical surface, depth of the activated cortical area, etc.) (Goldenholz et al., 2009; Huiskamp et al., 2010). Activity from cortical areas located close to the interhemispheric convexity appears to be clearly captured by MEG, while that from deeper sources (i.e. cingulate areas) is more difficult to detect (Goldenholz et al., 2009; Huiskamp et al., 2010). To the best of our knowledge, no study has specifically addressed the clinical added value of MEG in mesial ETLE. Still, several studies or case reports have highlighted the capability of MEG to detect interictal epileptic discharges from the mesial wall of the brain (Canuet et al., 2008; Garcia-Morales et al., 2009; Op de Beeck et al., 2011; Ibrahim et al., 2012; De Tiege et al., 2012; Jung et al., 2013; Gavaret et al., 2014; Heers et al., 2014; Murakami et al., 2016), with a clear impact on surgical management in some cases, i.e. detection of irritative zones not captured by conventional EEG or identification of a brain lesion in MRI-negative patients (figure 4) (Garcia-Morales et al., 2009; De Tiege et al., 2012; Jung et al., 2013; Murakami et al., 2016). These data underline that MEG is of great interest for patients with extra-temporal lobe epilepsy (De Tiege et al., 2012), and more particularly in MRI-negative patients (for a review, see e.g. Bagic, 2016) or patients with inconclusive conventional non-invasive presurgical evaluation (De Tiege et al., 2012). MEG can therefore provide added value for non-invasive presurgical workup of patients with such refractory mesial ETLE. However, MEG is expensive and therefore has limited availability which makes it difficult to implement in routine clinical practice.
Intracranial recordings
As electroclinical findings are often discordant in mesial ETLE and imaging cannot always pinpoint the ictal onset zone, invasive EEG recordings are needed for suspected focal epilepsies, even more so for tailoring resections in cryptogenic or MRI-negative cases (Chapman et al., 2005; Noe et al., 2013). Ictal SPECT can sometimes be unreliable or inconclusive due to rapid propagation in ETLE (Laich et al., 1997).
Precise depiction of the ictal onset zone and delineation of the epileptic cortex remains the cornerstone in achieving success after epilepsy surgery. When a focal seizure onset is suspected, but the epileptogenic zone cannot be clearly defined on the basis of semiology, EEG, MEG and advanced imaging, and invasive intracranial studies should be considered. Both depth electrodes, as well as subdural electrodes, can serve this purpose. Although interhemispheric subdural strip and grid electrodes have been reported to be associated with acceptable morbidity and allow for a mapping of adjacent functional areas, e.g. the mesial central cortex (Bekelis et al., 2012; Delev et al., 2015), one has to keep in mind that bridging veins can hinder appropriate placement of grids and therefore impede adequate delineation of the EZ.
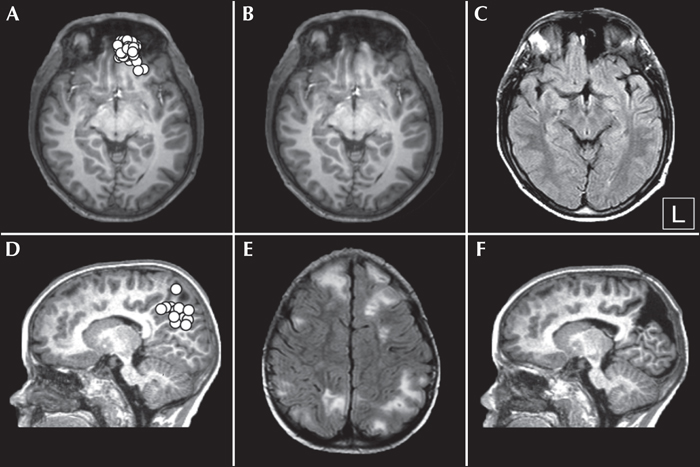
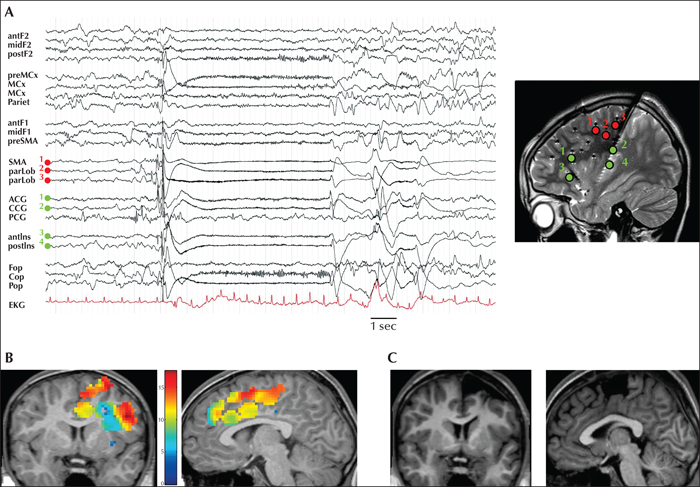
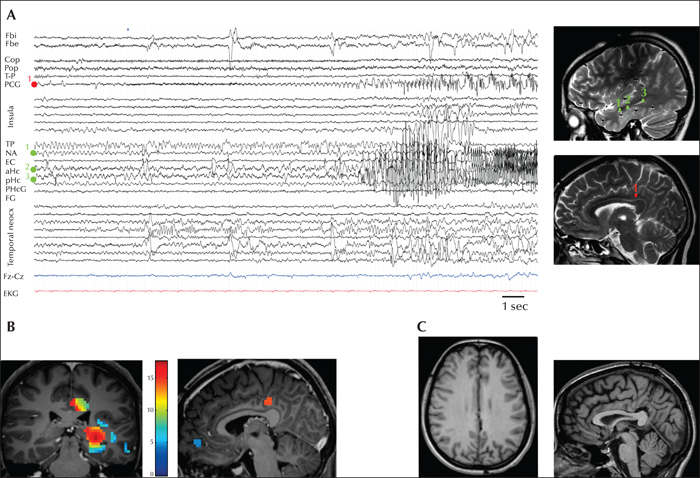
Stereo-electroencephalography (SEEG) is a safe method, associated with a lower level of risk relative to other invasive studies (Mullin et al., 2016). One of the advantages resides in the fact that the surgeon does not have to deal with bridging veins since a lateral approach is used to insert depth electrodes. Moreover, the same SEEG electrodes targeting the mesial cortex allow for coverage of intermediate sulci and (dorso-)lateral cortex, providing good spatial sampling, notably in frontal lobe epilepsies. SEEG also allows placement at the bottom of the sulci. Even before advanced imaging techniques, SEEG already provided a 3D approach to delineate the dysplastic cortex and therefore improved seizure outcomes after surgery (Chassoux et al., 2000). Defining an appropriate SEEG scheme requires meticulous review and analysis of all available data including semiology, video-EEG, neuronuclear imaging, MEG, fMRI, and neuropsychological assessment. In mesial ETLE, scalp EEG will often result in a mislocalization of the EZ. In such cases, other clinical information is critical to target the epileptogenic cortex. SEEG findings can furthermore be confirmed by the quantification of ictal high-frequency oscillations (David et al., 2011) between 60 and 100 Hz. Two illustrative SEEG cases are presented in figures 5 and 6.
Therapeutic challenges
Surgical considerations
On accessing the interhemispheric wall, one can encounter specific difficulties; bridging veins can impede access to the parasagittal cortex, and destroying or sparing eloquent cortex can respectively compromise functional or epilepsy outcome. Delineation of the extent of resection can be guided by different aids. Neuronavigation can be of great value in localizing a specific target area and determining the extent of resection, although brain shift can lead to inaccuracy. Intraoperative neurophysiology can furthermore guide a tailored resection in two ways. First, dysplastic epileptogenic cortex can lead to typical interictal epileptiform discharges with almost continuous spiking, which can serve as a guide in tailoring resections based on intraoperative electrocorticography (Palmini et al., 1995; Guerrini et al., 2015; Harvey et al., 2015). Secondly, intraoperative neurophysiology, with the use of motor and sensory evoked potentials as well as intraoperative electrical stimulation, can delineate the primary motor cortex and may lead to better post-operative seizure control (Neuloh et al., 2010). Preoperative functional MRI can already provide the surgeon with an estimated vicinity of the eloquent cortex to the presumed EZ. Some centres advocate the use of preoperative cortical mapping using navigated transcranial magnetic stimulation (TMS), e.g. in children when fMRI is not feasible. Furthermore, diffusion tensor imaging (DTI) can delineate important white matter tracts, such as the corticospinal tract in relation to the presumed EZ. One has to take into account the surgical morbidity related to the resection of a certain functional area and discuss the implications with the patient and their relatives before surgery. Surgical morbidity can result both from direct damage (resection and retraction) as well as from indirect vascular injury (bridging veins and interhemispheric arteries). Motor or speech deficits associated with SMA syndrome, which occur relatively frequently following frontomesial resections, can be anticipated and are generally associated with a good prognosis. Motor, sensory or visual deficits after resection of primary cortical areas tend to have a dismal prognosis.
Different surgical techniques have been described for interhemispheric lesions and tumours. Rotating the patient's head to the ipsilateral side with the mesial cortex facing upwards will allow the brain to fall down with gravity and obviates the need for retraction with spatulas. Regardless of the surgical technique, one has to avoid important traction on (eloquent) cortical areas and respect the course of bridging veins running along the mesial cortex into the sagittal sinus.
Outcome after mesial ETLE surgery
Seizure outcomes after ETLE surgery are generally reported with respect to the resected lobe, most frequently in the frontal lobe. Engel I outcomes for ETLE vary between 30% and 72% (Schramm et al., 2002; Kim et al., 2004; Dalmagro et al., 2005 ; Jeha et al., 2007; Binder et al., 2008; Elsharkawy et al., 2008; Lee et al., 2008; Binder et al., 2009; Elsharkawy et al., 2009; Jehi et al., 2009 ; Yu et al., 2009). ETLE surgery has been associated with inferior epilepsy outcomes, especially when considering MRI-negative cases (Smith et al., 1997; Mosewich et al., 2000; Chapman et al., 2005; Noe et al., 2013). Very few data are available on outcomes following resections in mesial extratemporal areas; when reported, overall, outcomes are good, with Engel IA outcome in over 60% of patients (mean follow-up: 5-9 years) (von Lehe et al., 2012; Alkawadri et al., 2013; Theys et al., 2017). In MRI-negative cases, 42% of mesial ETLE patients achieved Engel I outcome (Theys et al., 2017). This is comparable to a recent series of MRI-negative ETLE patients, of whom 38% had an excellent outcome after appropriate case selection (Noe et al., 2013).
Parasagittal resections are associated with a high rate of motor and speech deficits, but these are mostly transient. Transient neurological morbidity can be seen in up to 25% of patients with frontomesial epilepsy (Von Lehe et al., 2012). Permanent morbidity is mostly seen in central lobe and occipitomesial resections.
Conclusion
Mesial ETLE represents a challenging entity, both for the epileptologist as well as for the epilepsy neurosurgeon. Since the ictal onset zone can be clinically and electrographically silent and discordant findings are often present, a combination of imaging techniques with intracranial recordings are indispensable for obtaining good delineation of the EZ.
After extensive and invasive investigations, satisfactory outcomes can be obtained for this particular subgroup of extratemporal lobe epilepsies. For MRI-negative cases, this holds true for a selected subgroup of patients.
Disclosures
Tom Theys is a senior clinical investigator of FWO Flanders (FWO 1830717N), and is supported by Internal Funds KU Leuven (STG/15/053). Xavier De Tiège is a post-doctoral clinical master specialist at the Fonds de la Recherche Scientifique (FRS-FNRS, Brussels, Belgium). The MEG project at the CUB Hôpital Erasme is financially supported by the Fonds Erasme (Brussels, Belgium).
None of the authors have any conflict of interest to declare.