Epileptic Disorders
MENUColour vision in juvenile myoclonic epilepsy Volume 21, issue 2, April 2019
Juvenile myoclonic epilepsy (JME) is an idiopathic generalized epilepsy (IGE) syndrome that begins during puberty. It accounts for 20-27% of all IGEs, and about 5-11% of all epilepsies (Loddenkemper and Serratosa, 2006). Recent studies have shown that JME is a heterogeneous disease with a complex inheritance associated with various mutations (Sapio et al., 2015). Although the pathogenesis of JME has not been fully elucidated, new perspectives are developing based on EEG, new MR techniques, and nuclear medicine studies, such as SPECT and PET. Although this syndrome is accepted as a generalized epilepsy syndrome, some clinical and EEG features support local hyperexcitability of the cortex. The hyperexcitability of the motor system resulting from the degradation of cortical inhibitor pathways is responsible for the pathogenesis of JME (Zifkin et al., 2005).
JME patients are among those with the highest photoparoxysmal response (PPR) of all epilepsy patients (approximately 30%) (Verrotti et al., 2012). PPR is defined as an abnormal EEG response, also called “photosensitivity”, produced by intermittent photic stimulation (IPS). The pathophysiology of this phenomenon is thought to involve altered excitability of the visual pathways and regions of the occipital cortex in response to underlying genetic factors and flickering light (Verrotti et al., 2012; Strigaro et al., 2013). Also, it has been suggested that patients with PPR may have occipital hyperexcitability (Brigo et al., 2013). The PPR can be seen on EEG as spikes, or spike-and-wave or intermittent slow waves. PPR are divided into four subgroups according to the abnormal response to IPS:
- –spikes within an occipital rhythm;
- –parieto-occipital spikes with a biphasic slow wave;
- –parieto-occipital spikes with a biphasic slow wave and spread to the frontal region;
- –generalized spikes and waves or polyspikes and waves (Waltz et al., 1992; Doose and Waltz, 1993; Verrotti et al., 2012).
Although a relationship between visual system abnormality and PPR could not be established (Kasteleijn-Nolst Trenité, 1989), some recent studies have shown that PPR also characterizes some abnormalities in the visual system, such as altered visual contrast control and increased sensitivity to certain wavelengths. Some ocular abnormalities are also known to accompany PPR (Takahashi et al., 1999; Porciatti et al., 2000; Anyanwu and Ehiri, 2004).
We used the Farnsworth Munsell-100 Hue (FM-100 Hue) test, which discriminates between colours with high sensitivity, with the aim of revealing a possible alteration of colour perception responsible for photic sensitivity in patients with JME. In this respect, we aimed to reveal a possible relationship between photic sensitivity and colour vision function.
Materials and methods
The present study was designed as a prospective controlled study and conducted at our outpatient department. The study was approved by the Antalya Education and Research Hospital Ethics Committee.
Patients
The study group consisted of 51 JME patients (24 with and 27 without PPR) and 32 age/sex-matched healthy individuals. Data on seizure frequency and type, disease duration, antiepileptic drugs and doses, and neuroimaging findings of patients were obtained.
All patients were diagnosed with JME according to the recommendations issued by the International League Against Epilepsy (ILAE) Classification and Terminology Committee in 2010, based on seizure type, dominant seizure type, age at onset of seizures, EEG characteristics, and the “Diagnostic criteria for JME Class II” (see below) (Berg et al., 2010; Kasteleijn-Nolst Trenité et al., 2013).
Diagnostic criteria for JME Class II
- –Myoclonic jerks predominantly occurring on awakening.
- –Myoclonic jerks facilitated by sleep deprivation and stress and provoked by visual stimuli and praxis or generalized tonic-clonic seizures preceded by myoclonic jerks.
- –EEG showing a normal background and at least one interictal generalized spike or polyspike and waves with some asymmetry with or without myoclonic jerks.
- –No intellectual disability or deterioration.
- –Age at onset of between 6 and 25 years.
Each patient and healthy control underwent a complete ophthalmologic examination by the same physician who was uninformed of the EEG findings of the patients. Also, all patients underwent a best corrected visual acuity test, half-lamp biomicroscopy, intraocular pressure measurement, gonioscopy, dilate funduscopic examination, and refraction. Gonioscopy is the examination of the angle of the anterior chamber, and is used to identify eyes at risk of closure and detect angle abnormalities that could have diagnostic and therapeutic implications. It is a clinical technique used to examine structures within the anterior chamber angle and constitutes an essential diagnostic tool in everyday ophthalmic practice.
Inclusion criteria
- –Volunteer patients diagnosed with JME according to the ILAE.
- –Normal ophthalmologic and Standard Pseudoisochromatic Plates 2 (SPP2) examination.
Exclusion criteria
- –Other types of epilepsy other than JME.
- –Not volunteering.
- –Intellectual disability, psychiatric or systemic disease, or drug or substance use that may lead to deterioration of cognitive functions.
- –Abnormal SPP2 or ophthalmologic examination, ocular pathology (strabismus, nystagmus, and retinal pathology), or ocular surgery history.
- –A systemic disease that may affect the visual system, such as diabetes mellitus or hypertension.
EEG analysis
Photic stimulation was performed according to the “Updated European algorithm for visual stimulation in the EEG laboratory” (Kasteleijn-Nolst Trenité et al., 2012). All patients received standard 30-minute EEG recording, including four minutes of hyperventilation and five minutes of IPS using a Nihon Kohden/LS-703A Model stimulator with electrodes placed according to the international 10-20 system. IPS was performed under dim light conditions, with the patient in a vertical position, with simultaneous video recording. A circular reflector lamp was placed 30 cm away from the patient's nasion, and flashes with 0.7 Joules were produced. For IPS, the eyes were kept open for the first five seconds and then closed for at least seven seconds, before switching to the next frequency. The frequencies used for IPS were 1, 2, 4, 8, 10, 12, 15, 18, 20, 25, 40, 50, 60 Hz, and the filters were 0.5 and 70 Hz. The interictal EEG findings for each patient were evaluated by the same neurologist experienced in EEG photoparoxysms.
The Farnsworth Munsell-100 Hue test (FM-100 Hue)
The SPP2 test was used as the first colour vision examination to exclude congenital colour vision defects in patients and the control group. The FM-100 Hue test was then applied to the patients and control group, for whom the SPP2 test was error-free. The total error score (TES) as well as blue/yellow (B/Y) and red/green (R/G) error scores were calculated. The tests were performed on a black background, in front of a window, in daylight (between 12:00 pm and 2:00 pm), during summer and only on sunny days. In addition, the test was conducted in a quiet environment and without time constraints. TES and R/G and B/Y error scores were calculated using Microsoft Excel software according to the sequences performed by the patient and the control group, and the results were compared. The test practitioner, a physiologist, was blinded to the participants (patient or control group).
Statistical analysis
Data obtained from the study were evaluated using PASW 18 (SPSS/IBM, Chicago, IL, USA) software and p
Results
One of the 52 JME patients, who had congenital R/G vision impairment based on the SPP2 examination, was excluded from the study. Of the 51 JME patients included in the study, 36 (70.6%) were female and 15 (29.4%) were male, and the mean age was 26.7. The control group (n=32) consisted of 21 (65.6%) female and 11 (34.4%) male participants, and the mean age was 25.9.
Patients were divided into two groups according to the presence of photosensitivity on EEG. Thus, three groups were formed: the photosensitive JME group, the non-photosensitive JME group, and the healthy control group. In the photosensitive group, there were 17 (70.8%) females and seven (29.2%) males and the mean age was 28.6. In the non-photosensitive JME group, there were 19 (70.4%) females and eight (29.6%) males and the mean age was 24.96. There was no statistically significant difference in mean age or gender ratio between the photosensitive or non-photosensitive group and the control group (table 1A).
No significant difference in duration of epilepsy (table 1B) or total, B/Y or R/G error scores based on the FM-100 Hue test (table 2) was identified in patients with or without photosensitivity. However, total, B/Y, and R/G error scores were significantly higher in JME patients (independent of photosensitivity) than in the control group (table 3). Moreover, in the patient group, B/Y visual impairment was found to be significantly worse than R/G visual impairment (table 4).
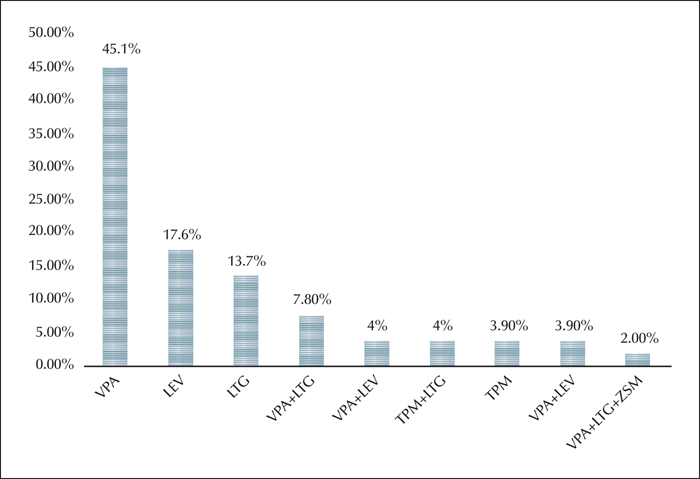
In the patient group, the most frequently used antiepileptic drugs (AEDs) were valproic acid (VPA) (mostly used alone), levetiracetam (LEV), lamotrigine (LTG), as well as polytherapy (figure 1). No significant difference in TES or B/Y and R/G error scores was identified according to the type of monotherapy used by the patients (table 5). Moreover, no differences in visual error scores were identified between patients receiving polytherapy and monotherapy, when analysed for each AED collectively (table 6) or separately (table 7)
Discussion
Although routine MR images of IGE patients have shown normal results, some studies have shown that various cortical and subcortical dystrophic neurons and various microscopic structural anomalies are present in these patients, which is defined as “microdysgenesis” (Meencke and Janz, 1984). Recently, this microdysgenesis in patients with JME has also been shown by voxel-based MRI (Woermann et al., 1999), and it is suggested that these structural abnormalities may be based on EFHC1 mutation in patients with JME (Suzuki et al., 2004). In addition, Anyanwu and Ehiri's study showed that ocular anomalies may be detected in patients with photosensitive epilepsy and that these abnormalities may affect visual acuity (Anyanwu and Ehiri, 2004). In the study conducted by Gomceli et al. (2016) on JME patients, the thickness of the peripapillary retinal nerve fibre layer of the superior quadrants for both eyes and choroid layer of the left eye was significantly greater, while the macular layer was significantly thinner in patients with photosensitivity compared to patients without photosensitivity. The choroidal layer provides metabolic support to the retina in the eye and is a vascular structure that provides blood flow to the external retinal layer. The changes in choroidal thickness and resulting metabolism and vascularisation may be related to photosensitivity, and these microstructural differences in the optic nerve may be the cause or the symptom of photosensitivity (Gomceli et al., 2016).
In this study, we investigated the relationship between photosensitivity and colour vision in JME patients in order to shed light on the pathophysiology of photosensitivity, which still remains unclear.
In the present study, we used the FM-100 Hue test, which is a very sensitive and widely used method to measure colour vision (Kinnear and Sahraie, 2002). We found no significant difference in total, B/Y or R/G visual acuity scores among JME patients with and without photosensitivity. However, when patients were compared with the healthy control group, the error scores were significantly higher in the patient group in all three categories, with the highest score for B/Y visual acuity. In the literature, colour vision function in epileptic patients taking AEDs was evaluated using the FM-100 Hue test and some abnormalities were detected (Tomson et al., 1988; Bayer, 1991; Bayer et al., 1995a, 1995b; Paulus et al., 1996). Colour vision functions of epileptic patients were compared with control group using the FM-100 Hue test before patients started medication and one year after carbamazepine (CBZ) or VPA were initiated. In both groups of patients, significant deterioration in error scores for colour vision was observed compared to the control group one year after the drugs were started. The reason for this disturbance is thought to be due to inhibition of voltage-gated sodium channel blockade by CBZ and voltage-gated sodium channel blockage and inhibition of T-type calcium channel or GABA receptor channels by VPA. It has been noted that these AEDs have an adverse effect on glutamate and other neurotransmitters in the retina, however, it is still unclear whether this adverse effect also occurs on photo- and bipolar receptors (Verrotti et al., 2004). Steinhoff et al. (1997) compared epilepsy patients receiving CBZ, VPA and a combination of CBZ, vigabatrin (VGB), gabapentin and topiramate treatments. VPA and VGB significantly impaired vision function in patients with both monotherapy and polytherapy compared to patients with other AEDs. It was speculated that this disturbance is associated with an increase in GABA in the brain caused by VPA and VGB. In another study, VGB, CBZ and placebo were compared in healthy volunteers. Deficits were detected in both areas (B/Y and R/G) in volunteers who were given CBZ and in the blue colour vision area in volunteers given VGB. It was reported that CBZ caused deterioration in both groups due to non-specific synaptic inhibition, and VGB caused deterioration due to GABAergic inhibition. It has also been suggested that the blue colour vision defect is a consequence of retinal damage and thus the GABAergic inhibition effect of VGB also occurs at the retina level (Mecarelli et al., 2001).
In our study, we firstly compared between monotherapy patients as the influence on this group may be due to medication usage. We then compared patients who were taking each drug as monotherapy with patients who received polytherapy, and finally all patients who received monotherapy and all patients who received polytherapy. However, no significant difference was found based on any of the comparisons. Therefore, no significant association was identified between type of AED, or mono- or polytherapy, and error score for colour vision. In the literature, no such side effects are reported following the use of LEV (Krakow et al., 2001). In our study, 17.4% of the patients were taking LEV monotherapy and their colour error scores were high. For this reason, we believe that this disturbance was not a side effect of the drug. As the JME patient group was not specifically assessed in the above-mentioned studies, this may account for the different results of our study.
The significant difference between the patients and control group raises the question “could colour vision impairment be due to the pathophysiology of JME?”. Koepp et al. (1997) investigated the distribution of C-11-labelled FMZ in the brain by PET and demonstrated increased cerebral cortex cBZ/GABA binding in patients with IGE and JME. This increase was linked to a presence of cortical hyperexcitability status and microdysgenesis in IGE patients. In another study, a total of 15 IGE patients (two of them with JME) were evaluated by magnetic resonance-spectroscopy (MR-S) and an increased level of GABA was detected in the occipital lobe (Simister et al., 2003). Hattingen et al. (2014) also found that GABA and precursor glutamine concentration increased in the frontal lobe and that GABA and N-acetyl-aspartate (NAA) concentration decreased in the thalamus in JME patients evaluated by MR-S. This result suggests the presence of a neurotransmission defect in this brain region. The fact that NAA is reduced in the grey matter of the thalamus supports the presence of a defect of GABAergic neurons. Finally, increased GABA and its precursor glutamine in the frontal lobe supports the congenital malformation of the thalamo-frontal neuronal network and the increase in GABAergic neuron density due to the number or structure of the receptors during corticogenesis. In the study of Cossette et al., mutations in the GABRA1 gene, encoding the subunit of the GABAA receptor, have been demonstrated in JME (Cossette et al., 2002; Cossette, 2010). Recently, new electrophysiologic findings have been detected by transcranial magnetic stimulation (TMS) in JME patients. These findings are believed to be due to a defective GABA-A mechanism as well as defective intracortical inhibition, resulting in a conserved GABA-B mechanism (Serafini et al., 2013). In conclusion, all these studies refer to a dysfunction in GABAergic mechanism in JME patients.
GABA is also the major neurotransmitter inhibitor in the retina and GABAergic transmission is involved in colour coding (Kupenova et al., 2010). This neurotransmitter, also acting as a major inhibitor in the central nervous system, plays an important role in retinal signal processing by being released from horizontal cells in the outer layer of the retina by a calcium-independent mechanism (Xu and Yang, 2000). This neurotransmitter has also been shown to regulate communication between horizontal cells and cone and bipolar cells (Xu and Yang, 2000). Recent studies support that mammalian horizontal cells are GABAergic (Vardi and Sterling, 1994). GABA and some other neurotransmitters have also been shown to be present in different types of amacrine cells in the retina and that interplexiform cells contain dopamine or GABA (Ryan and Hendrickson, 1987; O’Malley and Masland, 1989). GABAA and GABAC receptors are not localized to a particular synaptic region, suggesting that GABA may play a role in different pathways in the retina (Kupenova et al., 2010).None of the authors have
Therefore, GABAergic mechanisms appear to be a common pathway in both JME pathophysiology and colour vision physiology. Even though a dysfunctional GABAergic mechanism may play a significant role in the pathophysiology of JME in particular, rather than other epilepsy groups, more robust results are required based on a comparison between patients with JME and other epilepsy groups in future studies.
Conclusion
In the present study, although we were unable to demonstrate a correlation between photosensitivity and colour vision in JME patients, the error score for colour vision in JME patients was significantly higher than in the healthy control group. We hypothesise that the underlying cause of colour vision defects in JME patients may be due to the pathophysiology of the disease itself. Consequently, we believe that GABA dysfunction, which we consider to be involved in the pathophysiology of JME, also has an effect in the retina.
Disclosures
None of the authors have any conflict of interest to declare.