Environnement, Risques & Santé
MENUThe Maillard reaction and food safety: focus on acrylamide Volume 16, issue 1, January-February 2017
Figures
Tables
AgroParisTech
Inra
Université Paris-Saclay
91300 Massy
France
- Key words: Acrylamide, food contamination, risk assessment, Maillard reaction
- DOI : 10.1684/ers.2016.0952
- Page(s) : 31-43
- Published in: 2017
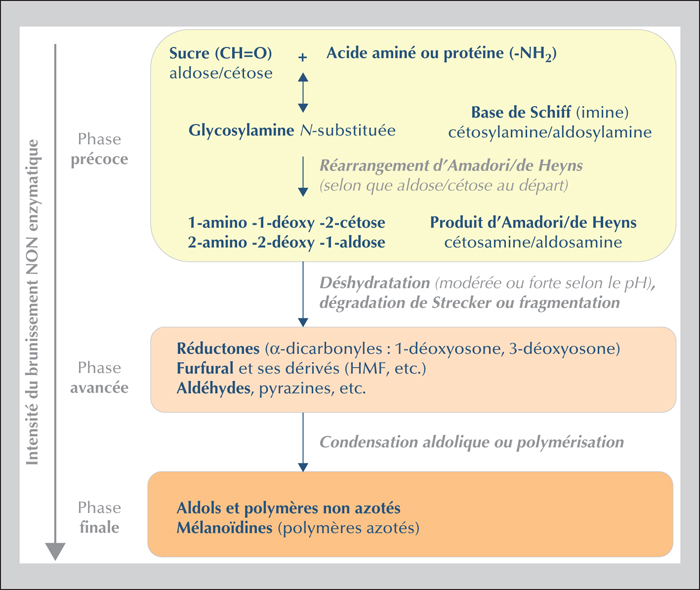
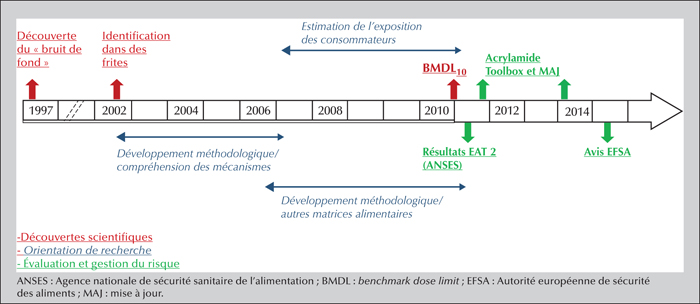
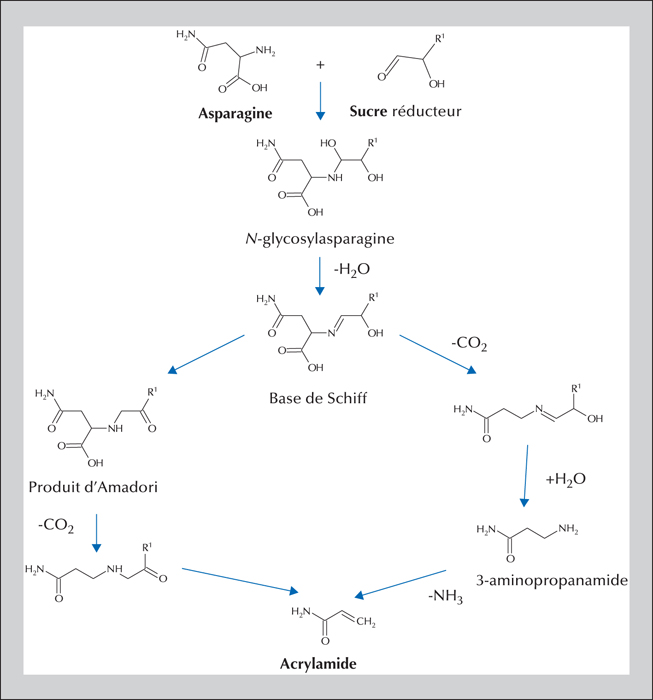
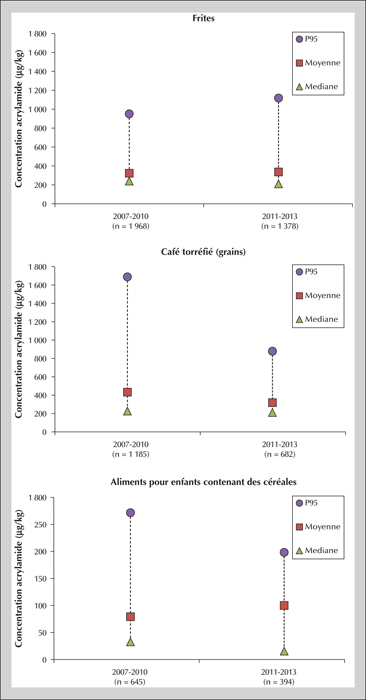
The Maillard reaction begins with a carbonyl group from a sugar and a free amino group from an amino acid or a protein and continues by different reactional pathways depending on the medium conditions. The compounds formed in situ in the food are frequently useful for the organoleptic quality of the food (aroma compounds), as well as its texture and color (browning). At the same time, however, other possibly toxic compounds may be formed: this is the case for acrylamide, a small polar molecule considered to be carcinogenic and genotoxic. Discovered in food in 2002, this molecule is mainly found in plant-based foods (e.g., processed cereals, French fries, roasted coffee). Numerous studies have sought to understand the mechanisms responsible for its formation in food along with the influencing factors. Tools are now available to the food industry to limit acrylamide formation. Elevated contamination levels have thus decreased, but mean levels have changed little since 2007. Consequently, dietary exposure of populations is relatively stable: a mean around 0.5 μg.kg-1(b.w.).d-1 for adults, and nearly double that for the most exposed consumers — children are about twice more exposed than adults. Risk assessment applying the margin of exposure approach leads to the conclusion that acrylamide is a risk to human health: the estimated margin of exposure values range from 50 to 500, while they should be over 10,000 to eliminate risk. Results from epidemiological studies do not confirm the risk (absence of significant association between acrylamide food exposure and cancer risk) but the lack of reliability of exposure assessment makes it impossible to rule out this risk.