Virologie
MENUCRISPR-Cas: the bacterial immunity that supports diagnostic in virology Volume 26, issue 4, Juillet-Août 2022
- Key words: CRISPR, Cas9, Cas12a, Cas13a, Diagnostic, Virology
- DOI : 10.1684/vir.2022.0966
- Page(s) : 303-13
- Published in: 2022
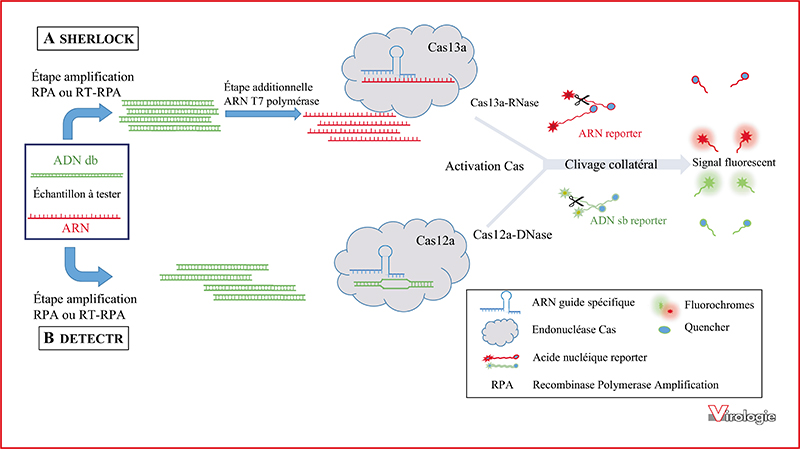
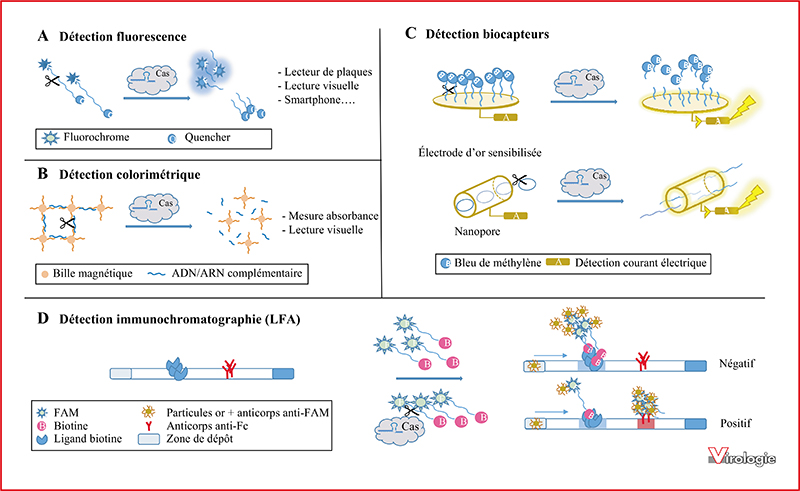
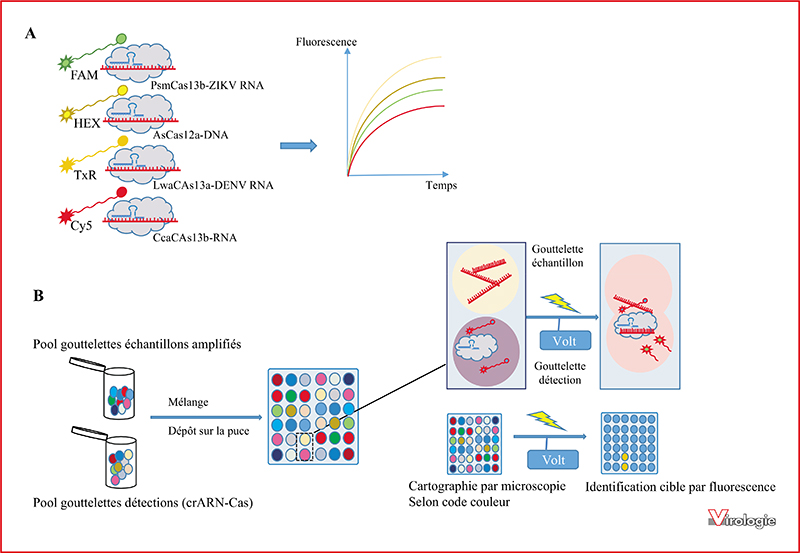
CRISPR-Cas is an adaptive immune system that prevents bacteria and archea from nucleic acids invasion such as viral genomes. The ability of the CRISPR-Cas technology to effectively and precisely cut a targeted genomic DNA region was exploited to develop powerful genome editing tools that were adapted for a wide range of applications, revolutionizing biological sciences. The CRISPR-Cas system consists of a Cas endonuclease triggered by a RNA guide for highly specific cleavage of targeted DNA or RNA sequences. In addition to the target specific cleavage, some Cas enzymes, including Cas12a and Cas13a, display a collateral trans-cleavage activity that allows the cleavage of all surrounding single-stranded nucleic acids. These biosensing activities of CRISPR-Cas systems, based on target specific binding and cleavage, are promising tools to develop accurate diagnostic methods to detect specific nucleic acids. CRISPRCas could therefore be used to diagnose a wide variety of diseases. In the current review we propose to describe the more significant advances for virus detection based on CRISPR-Cas systems.