Cahiers Santé Médecine Thérapeutique
MENUNew antibiotics for multi-drug resistant bacterial strains Volume 31, issue 3-4, Mai-Août 2022
Figures
-
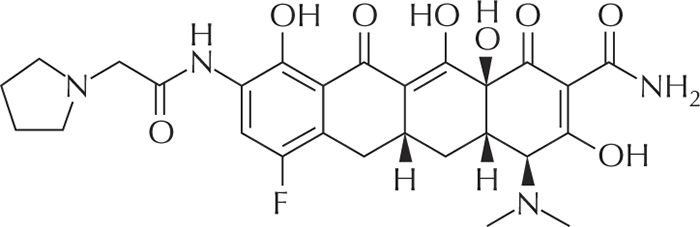
Figure 1 -
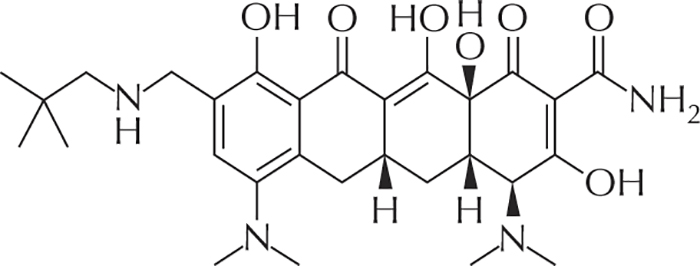
Figure 2 -

Figure 3 -
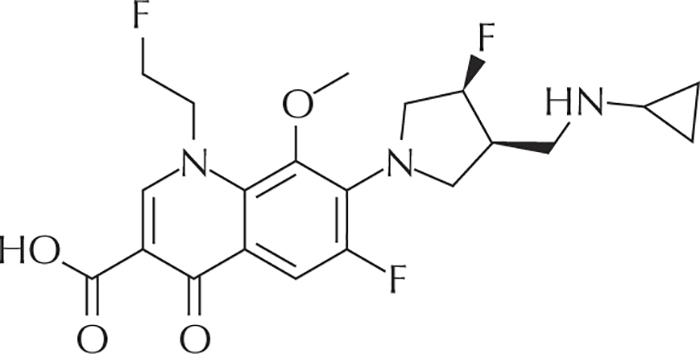
Figure 4 -
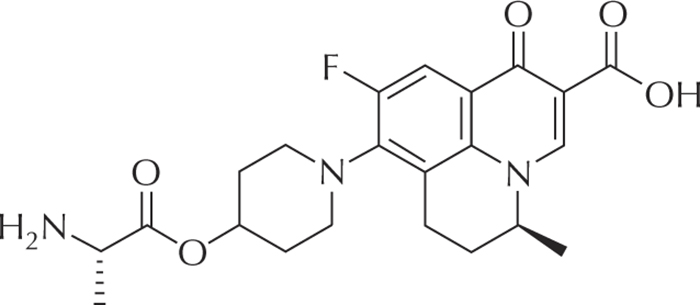
Figure 5 -
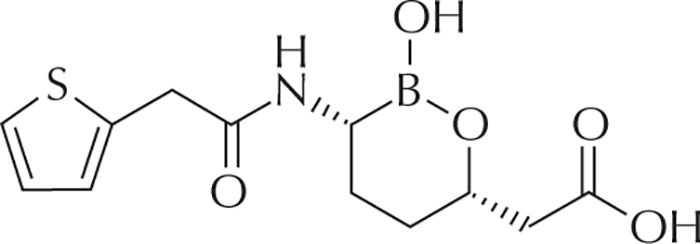
Figure 6 -
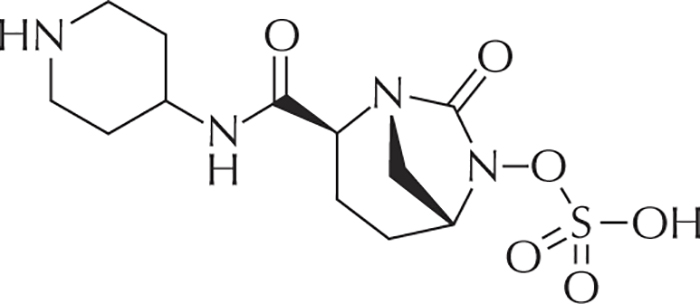
Figure 7 -
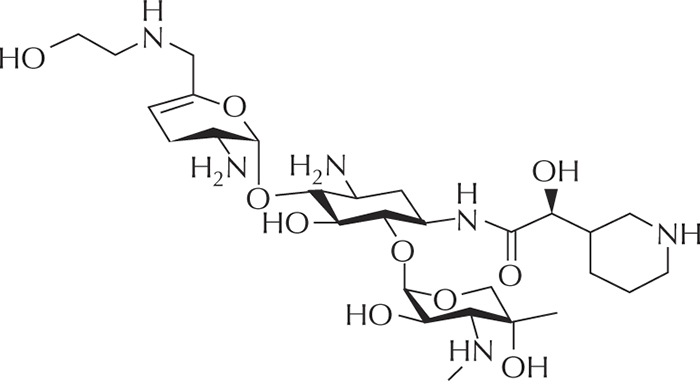
Figure 8 -
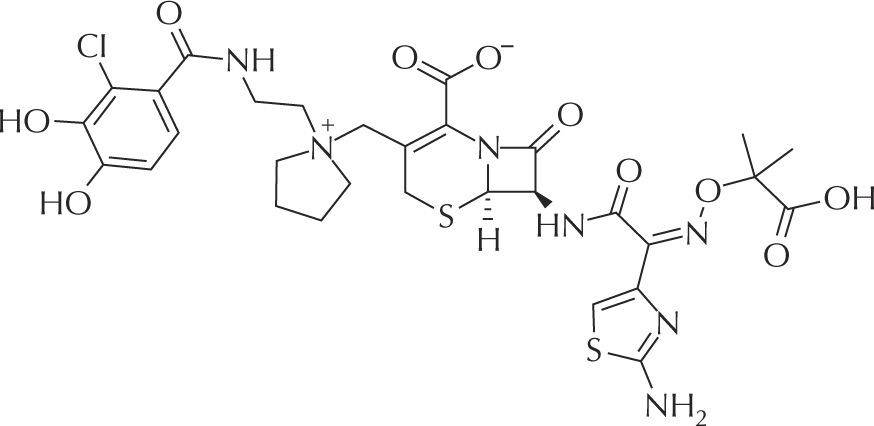
Figure 9 -
Figure 10 -

Figure 11
Tables
- Key words: new antibiotics, priority pathogens, World Health Organization (WHO)
- DOI : 10.1684/sanmt.2023.152
- Page(s) : 208-18
- Published in: 2022
Antimicrobial resistance is a major health problem. It increases mortality and morbidity and puts a strain on health systems. The discovery of new antibacterial drugs with activity against multidrug-resistant bacteria presents economic and scientific challenges. In 2017, the World Health Organization (WHO) published a list of 12 priority antibiotic-resistant pathogens and began to critically analyse the antibacterial pipeline. This review analyses the 'traditional' and 'non-traditional' antibacterial agents and modulators that have received marketing authorisation between 2017 and 2022 and the most promising new molecules still in clinical investigation (phase III), apart from treatments specifically active against Clostridium difficile and Mycobacterium tuberculosis. Since 2017, 11 new antibacterial drugs have been approved worldwide, but only vaborbactam belongs to a new antibacterial class. Cefiderocol, a cephalosporin derivative, is also innovative. It incorporates an iron-chelating siderophore that facilitates the entry of gram-negative bacterial cells. In total, there were 11 antibacterial agents in Phase III and IV clinical development under regulatory review. For so-called “non-traditional” approaches to antibacterial therapy, most candidates are undergoing clinical evaluation as adjuvant therapies in combination with “standard of care” antibiotics. To date, only three non-traditional antibacterial agents have been approved, all of which are monoclonal antibodies.
