Médecine de la Reproduction
MENUMitochondrial egg therapies Volume 24, issue 2, Avril-Mai-Juin 2022
- Key words: mitochondria, embryo, fertility, mitochondrial replacement therapy (MRT)
- DOI : 10.1684/mte.2022.0889
- Page(s) : 219-28
- Published in: 2022
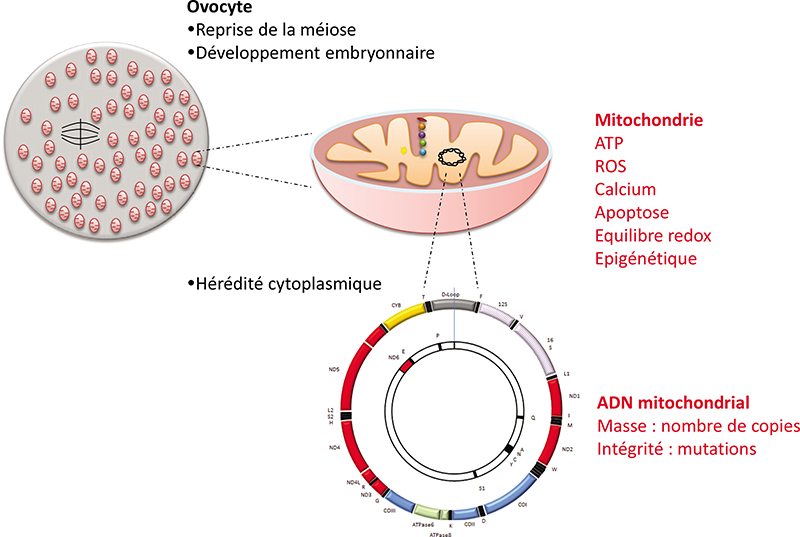
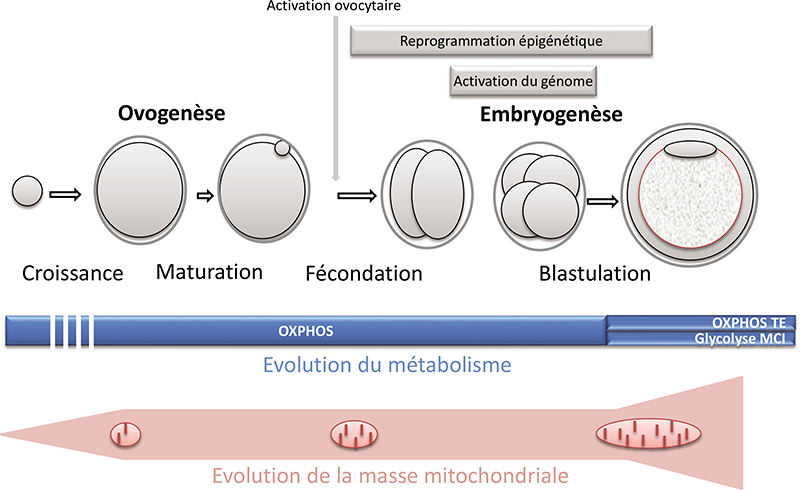
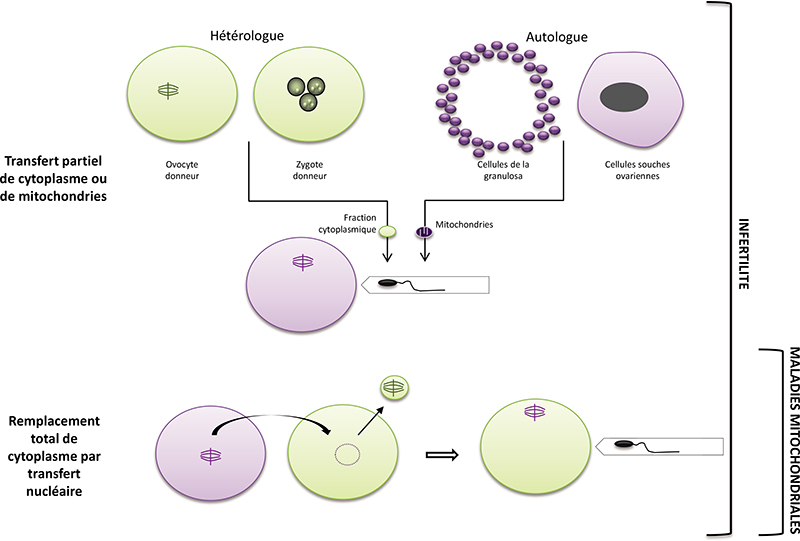
In the embryo, mitochondria are exclusively inherited from the oocyte. They play essential roles in pre-implantation development in mammals: energy production, regulation of redox and calcium homeostasis, production of substrates necessary for epigenetic reprogramming. A decrease in their quantity has been associated with developmental defects. Moreover, mitochondrial DNA mutations are a cause of severe and incurable diseases transmitted by the mother when the mitochondrial DNA of the oocyte carries certain mutations. Mitochondrial replacement therapies have been proposed to supplement (partial cytoplasm transfer) or replace (total cytoplasm transfer) oocyte mitochondria and to alleviate certain infertilities or to avoid the transmission of mitochondrial DNA diseases. This article reviews the attractive but highly controversial use of these techniques. In any case, they are likely to disrupt mitochondrial homoplasmy and the necessary dialogue between the nuclear and mitochondrial genomes, two properties that naturally result from the mode of maternal transmission of mitochondria and whose regulation remains insufficiently understood to date. The use of agents stimulating mitochondrial activity or biogenesis could, in the case of infertility, constitute an alternative to these techniques. Further research is needed to assess the possible long-term effects of these different strategies.