Médecine de la Reproduction
MENUAdvances in spermatogenesis approaches in vitro Volume 24, issue 2, Avril-Mai-Juin 2022
- Key words: spermatogenesis in vitro, human, fertility preservation, organotypic culture
- DOI : 10.1684/mte.2022.0884
- Page(s) : 145-51
- Published in: 2022
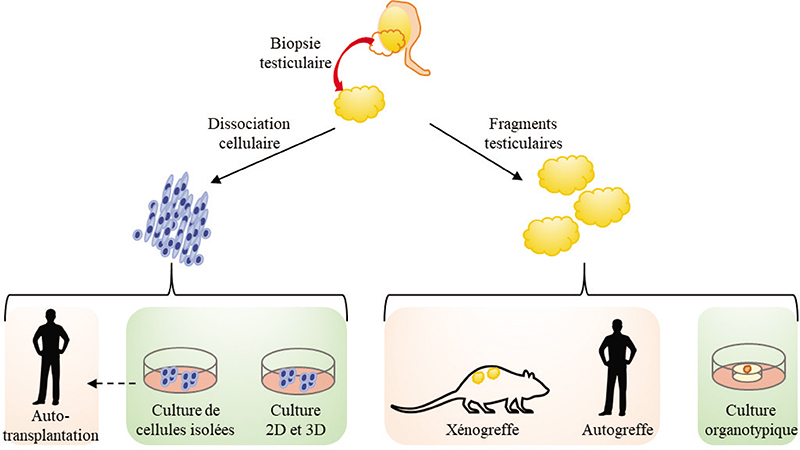
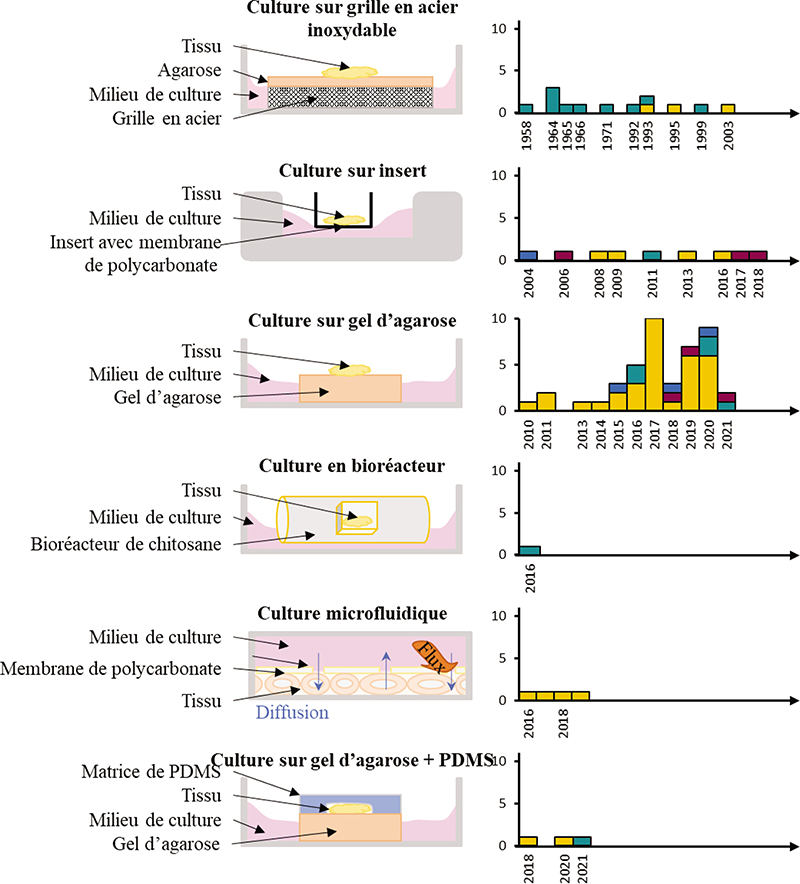
In order to restore fertility in patients with preserved testicular tissue from patients cured of paediatric cancer in particular, different approaches of in vitro maturation of (pre)pubertal testicular tissue have emerged in order to reproduce in vitro the complete process of spermatogenesis and have continued to evolve over the last 50 years. The aim of in vitro spermatogenesis is to generate in vitro spermatozoa from isolated spermatogonial stem cells (SSCs) or intact fragments of testicular tissue. Isolated SSCs, obtained after enzymatic and/or mechanical dissociation of testicular tissue, can be grown in liquid medium (twodimensional culture) or within a matrix (three-dimensional culture). The culture of testicular tissue fragments or organotypic culture, most often performed on agarose gel in gas-liquid interphase, allows the preservation of the interactions of SSCs with their microenvironment. Progressively, different models are emerging with the aim of mimicking the physiological conditions of spermatogenesis, particularly its spatio-temporal organisation. This research has led to the production of functional spermatozoa and viable and fertile offspring in mice. Translational research has been set up to transfer these technologies to human testicular tissue.