Hématologie
MENUNeutrophil extracellular traps: key drivers of severe COVID-19 Ahead of print
Figures
- Key words: Covid-19, neutrophil, respiratory failure, inflammation, thrombosis
- DOI : 10.1684/hma.2021.1704
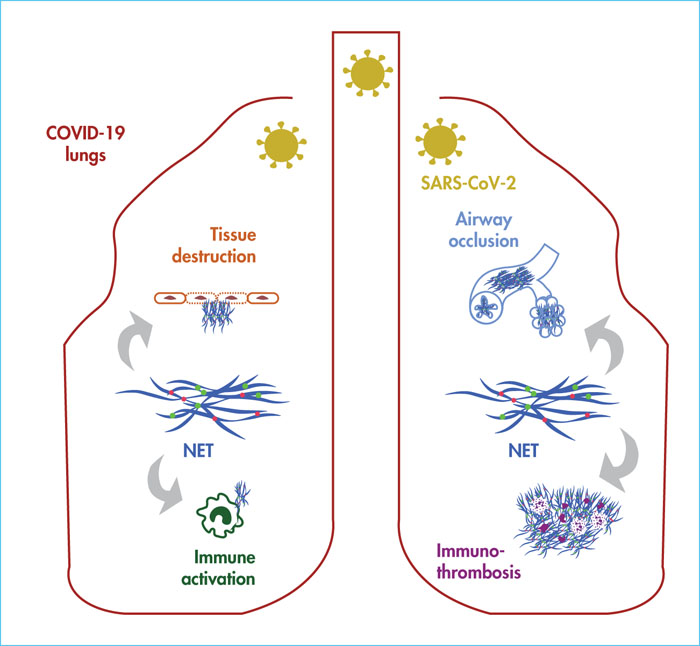
In severe COVID-19, hyperinflammatory tissue-damaging, thromboembolic or immunothrombotic responses triggered by SARS-CoV-2 are major causes of respiratory failure and death. Neutrophil extracellular traps (NETs), released by activated neutrophils during a process known as “NETosis”, can be formed in the lungs upon infection with respiratory viruses. They have the ability to promote lung damage, thrombosis and fibrosis, three cardinal features encountered in severe COVID-19. In a recent study, we sought to understand how NETosis could be related to lung immunopathological changes associated with fatal cases of the disease. We assessed whether NET structures could be identified in post-mortem lung biopsies from COVID-19 patients, and whether they were located in particular lesions and microanatomical lung compartments. We performed immunofluorescence staining of myeloperoxidase, citrullinated histone H3 and nuclear acid (DAPI) on sections of paraffin-embedded lung biopsies from four COVID-19 patients who succumbed to COVID-19 and from four patients who died from a cause unrelated to COVID-19. The former four patients represented prototypical severe and fatal cases of COVID-19, characterised by pneumonia and fatal respiratory distress associated with signs of systemic inflammation, neutrophilia and coagulopathy. NETs were uniquely detected in the lungs of all the COVID-19 patients. Detailed histopathological analysis revealed widely distributed NET-infiltrating areas encompassing several lung compartments, including arteriolar microthrombi, neutrophil-rich inflammatory areas of the lung interstitium, as well as the alveoli or bronchioles, where they often co-localised with occluding fibrin-rich deposits. Another study published simultaneously to ours provides the first experimental evidence of causality between NETosis and lung injury in severe COVID-19. However, the possible involvement of NETs in thrombogenesis remains to be addressed. Nevertheless, these data support the hypothesis that NETs may represent drivers of COVID-19-associated severe pulmonary complications and suggests that NET-targeting approaches could represent potential future avenues for the treatment of uncontrolled tissue-damaging, thrombotic or fibrotic responses to SARS-CoV-2.