Hématologie
MENUFunctional and clinical aspects of the anti-hemophilic bispecific antibody emicizumab Volume 26, issue 6, Décembre 2020
- Key words: Hemophilia A, factor VIII, emicizumab, coagulation, bispecific antibody
- DOI : 10.1684/hma.2020.1604
- Page(s) : 328-42
- Published in: 2020
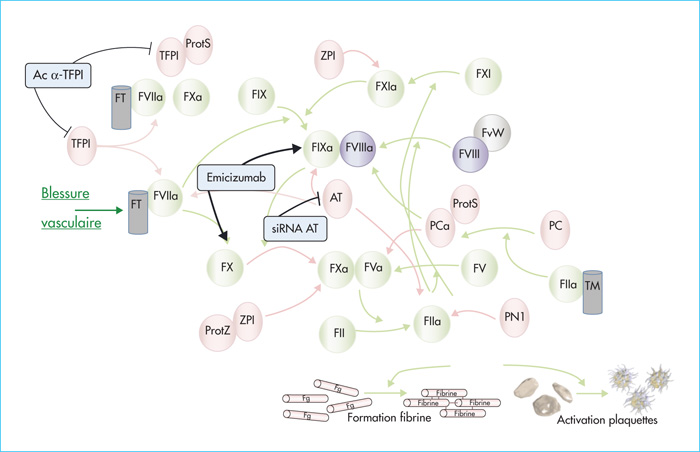
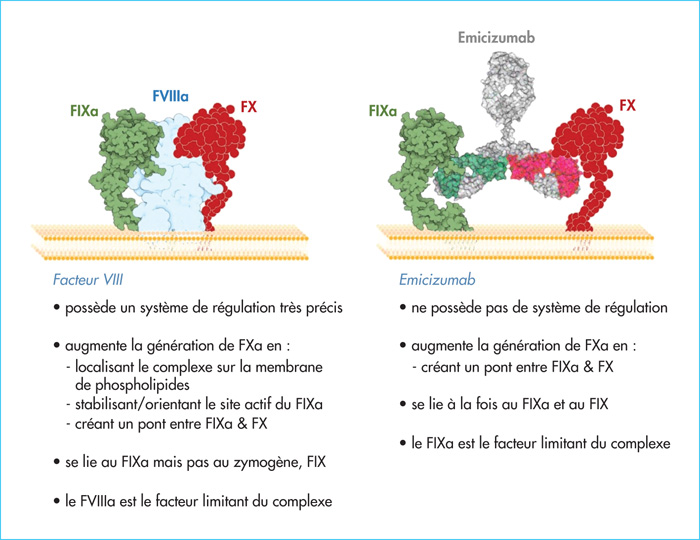
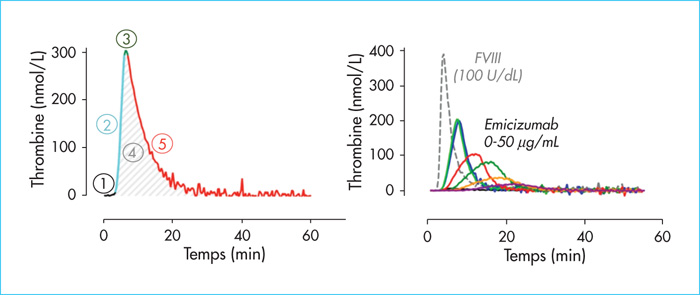
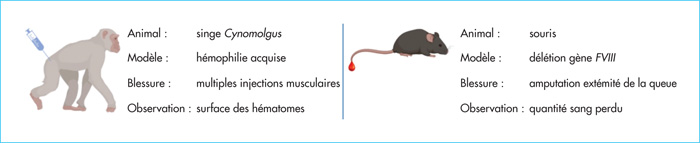
Coagulation factor VIII (FVIII) functions as a non-enzymatic cofactor for the enzyme factor IXa (FIXa) in the activation of factor X (FX). The functional deficiency of FVIII is associated with a severe bleeding disorder known as hemophilia A, affecting 1-2 per 10,000 male births. Bleeding complications have long been managed via FVIII substitution therapy, preferably on a prophylactic basis. Due to limitations of this approach (i.e., the need for frequent intravenous infusions, development of neutralizing antibodies), novel treatment options for hemophilia A have been evaluated, including the use of the bispecific antibody emicizumab. Emicizumab is designed to mirror FVIII cofactor function by promoting the spatial approximation of FIXa and FX. In this review, we will discuss functional and clinical aspects of emicizumab. We will compare its mode of action to that of FVIII and consider the implications for laboratory monitoring of this antibody. In addition, we highlight the clinical data obtained in the pivotal HAVEN-trials, which have shown a clear clinical benefit by strongly reducing the occurrence of spontaneous bleeding episodes. Finally, we discuss some of the adverse effects that have been reported and also the potential use of emicizumab beyond patients with severe hemophilia A.
![]() This work is licensed under a
Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License
This work is licensed under a
Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License