Epileptic Disorders
MENUSensitivity of magnetoencephalography as a diagnostic tool for epilepsy: a prospective study Volume 22, issue 3, June 2020
Epilepsy is a debilitating neurological disorder with a broad spectrum of seizure semiology and frequency, ranging from abnormal sensations to complex and long-lasting convulsions that occur less than once a year to up to several times a day (Clarke et al., 2017). Therefore, epilepsy has a significant impact on daily life and significant social and occupational implications. In The Netherlands, about 180,000 people suffer from epilepsy (Centraal Bureau voor de Statistiek, 2016; NIVEL Zorgregistraties, 2017). The worldwide prevalence is approximately 50 million people and an estimated 2.4 million people are newly diagnosed with epilepsy every year (World Health Organization, 2018). This highlights the great importance for accurate and effective diagnosis.
Current guidelines (Werkgroep richtlijn ‘Epilepsie’, 2016; The National Institute for Health and Care Excellence, 2017) state that a medical specialist with training and expertise in epilepsy should be able to diagnose epilepsy. A detailed history needs to be obtained from the patient and, if possible, from a relative who has observed the seizure(s). The next step is to perform electroencephalography (EEG). Based on the detection of interictal epileptiform discharges (IEDs), a routine EEG can support the diagnosis of epilepsy and can help to determine the seizure type and epilepsy syndrome. If a routine EEG does not contribute to diagnosis or classification, an EEG following sleep deprivation (EEGsd) can be performed. EEGsd shows interictal abnormalities in approximately 40% of patients with epilepsy whose routine EEG was normal (Giorgi et al., 2013; Geut et al., 2017). An alternative to EEGsd could be long-term video or ambulatory EEG. Another component in the diagnostic process is neuroimaging, preferably magnetic resonance imaging (MRI), which may lead to the identification of structural abnormalities that can cause epileptic seizures. All in all, the diagnosis of epilepsy is ultimately based on the final clinical diagnosis by the treating physician which is considered as the gold standard.
The above-mentioned diagnostic process can be lengthy and stressful for the patient. Magnetoencephalography (MEG) is a technique that non-invasively measures brain activity by recording extra-cranial magnetic fields using superconducting quantum interference devices (SQUIDS) (Baillet, 2017). At present, MEG is mainly used in the preoperative evaluation of epilepsy patients (De Tiège et al., 2017). However, previous studies have shown that MEG is more sensitive to epileptiform discharges than EEG (Ossenblok et al., 2007). In a prospective study of 51 patients suspected to have epilepsy with inconclusive routine EEG, MEG provided additional information leading to the diagnosis of epilepsy in 63%. In the same patients, a diagnostic gain of 57% was found for EEG following sleep deprivation (Colon et al., 2009). The long-term follow-up of this study showed that epileptiform abnormalities on MEG tend to be more robust long-term predictors, as the diagnostic gain with MEG was 61%, relative to the most recent diagnosis, compared to 50% for EEGsd (Colon et al., 2017). In another study in which patients with suspected epilepsy and repeatedly normal EEG recordings were investigated, simultaneously recorded MEG and EEG demonstrated a sensitivity of 41%, with MEG providing 18% additional sensitivity. Therefore, MEG provides additional diagnostic information in patients suspected of having epilepsy (Duez et al., 2016).
Furthermore, MEG is more patient-friendly as the recording time is limited, diurnal sleep-wake rhythm is not disturbed, and no attachment of electrodes is needed. This advantage is only valid if MEG is recorded without simultaneous EEG.
The objective of this study was to determine the benefit of routine MEG with regard to diagnostic gain relative to frequently used examinations such as routine EEG, EEGsd or 24-hour EEG, when available. We hypothesised that the sensitivity and specificity of routine MEG would be comparable to or higher than that for the above-mentioned EEG examinations. Furthermore, a diagnostic gain using routine MEG may be expected for epileptiform discharges that are not apparent on EEG.
Methods
Study design
For this prospective trial, patients were included from two centres in The Netherlands (Academic Centre for Epileptology Kempenhaeghe/Maastricht University Medical Centre [MUMC+], Heeze and Elisabeth-Twee Steden Hospital, Tilburg). After referral for routine EEG, patients were offered additional routine MEG (performed at the Amsterdam University Medical Centre, Vrije Universiteit Amsterdam) besides frequent examinations such as routine EEG, additional EEG (EEGsd or 24-hour EEG) if needed, and MRI. The study was approved by the Medical Ethics Committee of the Vrije Universiteit Medical Centre (VUmc), Amsterdam, The Netherlands (NL43357.029.13).
Participants
Consecutively referred patients were screened for participation in this study. Patients were referred from peripheral hospitals in the south of The Netherlands by the treating physician who requested an expert opinion. Inclusion criteria were:
- –a clinical suspicion of epilepsy;
- –six years of age or older;
- –and ability to co-operate.
Patients were excluded based on:
- –a high suspicion of non-epileptic seizures;
- –a pacemaker or (intracranial) metals;
- –and not being able to meet the mild physical or psychological criteria for MEG recording.
Patients were included between August 2013 and March 2016. Written informed consent was obtained from all patients and, if
Test methods
Index test: routine MEG
Routine MEG recordings, not combined with EEG or video recordings, were performed at the MEG centre of Amsterdam University Medical Centre, Vrije Universiteit, Amsterdam, The Netherlands. The MEG recordings, including preparation, took approximately 60 minutes. Six electrodes were attached to the skin for electrocardiography (ECG) and electrooculography (EOG) recordings. A three-dimensional digitizer (Fastrak; Polhemus, Colchester, VT, USA) digitized the positions of four or five head-localization coils and the scalp outline, which consists of roughly 500 points. The points on the scalp surface were used for correlation with anatomical MRI of the patient through surface-matching. During the recording, patients were in supine position with their head placed in the MEG helmet. In line with the protocol for routine EEG recordings, patients were asked to perform several tasks:
- –open and close their eyes;
- –make a fist with the left or right hand;
- –sigh (hyperventilation);
- –and relax.
For recordings, a 306-channel whole-head MEG system with 102 magnetometers and 204 gradiometers (Elekta Neuromag Oy, Helsinki, Finland) was used. Data sampling frequency was set at 1,250 Hz and the data were filtered using an online 410-Hz anti-aliasing filter and a 0.1-Hz high-pass filter. The head-localization coils were activated continuously to record the head position relative to the MEG sensors. The raw data were spatially filtered to remove artefacts using the temporal extension of Signal Space Separation (tSSS) as implemented using MaxFilter software (Elekta Neuromag Oy; version 2.1).
The MEG recordings were visually assessed by a neurologist specialized in epilepsy. The assessor was aware of the clinical data and the MRI, if available, but not of the results of the EEG recordings. The results of the routine MEG recordings were categorized as “epileptiform” (multiple spikes
Reference standard: EEG, EEGsd or 24-hour EEG, and MRI
EEG recordings were performed according to protocol. Recording time was one hour for routine EEG and two and a half hours for EEGsd. Sleep deprivation was obtained in the inpatient ward, from midnight to approximately seven o’clock in the morning. During EEG recordings, patients were asked to perform the same tasks as during MEG recordings. A 24-hour EEG was performed either in the in- or outpatient clinic, and recorded in the patients’ own surroundings in the latter.
In Kempenhaeghe, EEG recordings were made using BrainRT for the first half of the study. Sample frequency was set at 250 Hz and high-pass filtering at 0.0713 Hz was applied. For the other half of the study, Micromed was used with sampling at 256 Hz and a high-pass filtre at 0.15 Hz. The 10-20 system was used throughout the whole study. When appropriate, extra electrodes of the 10-10 system were added. In the Elisabeth-Twee Steden Hospital, BrainRT was used during the entire period of the study using the same protocol as that in Kempenhaeghe.
EEGs were visually assessed independently by clinical neurophysiology technicians and neurologists specialized in epilepsy. The assessors were aware of the clinical data and the MRI, if available, but not of the results of the index test. Results of the EEGs were categorized as “epileptiform” (multiple spikes
Due to the significant variation in possible sequence parameters for brain MRI, strict rules were applied as to whether available MRI scans from referring centres were used. If MRI was performed in the referring centre, the images and reports were requested. Radiologists specialized in epilepsy in Kempenhaeghe or Elisabeth-Twee Steden Hospital reassessed the images when available. If not, the treating physician checked whether the MRI report from the referring centre was sufficient. When the assessing specialist concluded that the report was insufficient for the purposes of this study, or when no previous MRI was available, a new MRI was performed in Kempenhaeghe or Elisabeth-Twee Steden Hospital. In these centres, a 3T MRI scan (3.0 T Achieva, Philips, Best, The Netherlands) was performed and analysed by a neuroradiologist specialised in epilepsy, who was aware of the clinical data and, in some cases, of the EEG results. The 3T MRI sequences included 3D-T1 (TR: 8.1 ms; TE: 3.7 ms; voxel: 1 × 1x1 mm), T2 (TR: 3000 ms; TE: 80 ms; voxel: 0.5 × 0.5 × 0.5 mm), T2* (TR: 777 ms; TE: 16 ms; voxel: 0.9 × 1.1 × 5 mm), IR (TR: 120 ms; TE: 10 ms; TI: 400 ms; voxel: 0.4 × 0.6 × 2 mm) and FLAIR (TR: 8000 ms; TE: 50 ms; TI: 2400 ms; voxel: 1.1 × 1.1 × 0.5 mm).
The MRI results were checked and considered to be positive if an abnormality that could explain the epileptic seizures was present.
The ultimate diagnosis of epilepsy was based on the final diagnosis by the treating physician, which was based on all available clinical data and test results at the end of the diagnostic process. In some cases, it remained uncertain whether the patient suffered from epilepsy or not. Therefore, the final diagnosis by the treating physician was categorized as “epilepsy”, “uncertain”, or “no epilepsy”.
Statistical analysis
Primary outcomes
For analysis, data from EEGsd and 24-hour EEG were gathered under the combined group of “additional EEG”, providing at least one sleep cycle was present for either; during our clinical practice, patients are offered the choice between these two when an additional EEG is needed for the diagnostic process. The results of the EEG and final clinical diagnosis were categorized into three groups, and MEG data into four groups. To calculate the percentage of concordance between each modality and the clinical diagnosis, the groups were pooled. For sensitivity, the final clinical diagnosis of “no epilepsy” and “uncertain” were pooled into one group, as were EEG results indicating “no epileptiform abnormalities” and “uncertain”. The opposite was done for specificity: “epilepsy” and “uncertain” were pooled into one group for the final clinical diagnosis, as were the EEG results for “epileptiform” and “uncertain”. Pooling was carried out to prevent overestimation of the diagnostic value for each modality. The derived sensitivity and specificity of routine EEG and routine EEG in combination with additional EEG(s) were compared to routine MEG using the McNemar Test with IBM SPSS Statistics, version 24. When data were missing, either due to withdrawal or loss to follow-up, patients were excluded from analysis. All statistics were performed by an independent researcher with supervision by the leading epileptologist in the study.
Secondary outcome
An additional analysis was performed in all patients with a lesion on MRI that could possibly cause the epileptic seizures. If the localization of the lesion matched the final clinical diagnosis, lobular localization was determined based on routine MEG.
Sample size calculation
Prior to this trial, a sample size was calculated. We aimed to detect a clinically relevant difference in sensitivity of at least 12.5% between routine MEG and routine EEG. A dropout rate of 20% was estimated. Assuming alpha at 0.05 and power at 0.85, an appropriate sample size of 341 patients was calculated.
Results
Participants
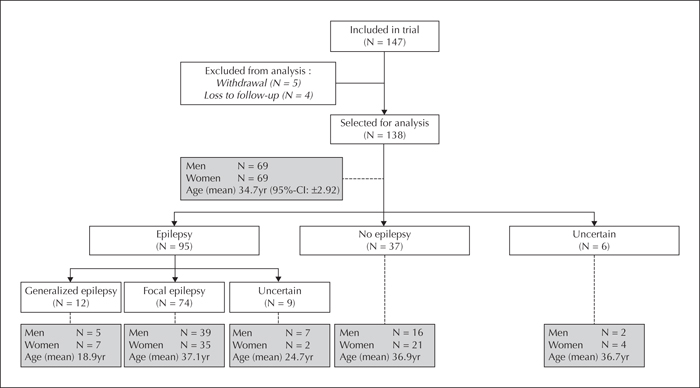
During the study, the protocol at Kempenhaeghe changed and no routine EEGs were performed for referrals from peripheral hospitals. It was therefore impossible to further include participants, thus the intended sample size was not achieved. Up to March 2016, a total of 147 patients were included. Five of them withdrew from participation and four patients were lost to follow-up. Analyses were therefore completed with a study population of 138 patients. Baseline characteristics are shown in figure 1. According to the final clinical diagnosis, 95 patients had epilepsy, of whom 12 were diagnosed with generalized epilepsy and 74 with focal epilepsy. The syndrome diagnosis was uncertain in the remaining nine patients. Thirty-seven patients were considered not to have epilepsy and eight of these were diagnosed with psychogenic non-epileptic seizures (PNES). In six patients, the final clinical diagnosis was uncertain (figure 1).
Overall, 111 patients underwent either one (n = 109) or two (n = 2) additional EEGs and in 27 patients only a single routine EEG was performed. In the latter, eight routine EEGs were categorized as “epileptiform”, 17 as “no epileptiform abnormalities” and two were “uncertain”. Diagnosis was congruent with these results in 16 patients, including all eight patients with routine EEG categorized as “epileptiform”.
The median timeframe in which routine EEG, additional EEG and routine MEG were performed was 30 days, with a minimum and maximum of one and 326 days, respectively. One extreme value was excluded. During this timeframe, no clinical interventions were performed and no adverse events due to the index test or other examinations were reported.
Test results
Primary outcomes
Routine EEG showed a sensitivity of 31.6% and a specificity of 100%, with a positive predictive value (PPV) and negative predictive value (NPV) of 93.8% and 35.9%, respectively (table 1). The results of routine EEG and, if available, additional EEG were combined for analysis. If one of the EEGs was assessed as “epileptiform”, the combined result was considered “epileptiform”. If both showed no epileptiform abnormalities, they were categorized as “no epileptiform abnormalities”. If both were uncertain, or one was uncertain and one showed no epileptiform abnormalities, the combined result was “uncertain”. A sensitivity of 52.6% and specificity of 97.3% was found for the combined EEGs, with a positive predictive value of 94.3% and a negative predictive value of 45.6% (table 2). All 138 patients underwent routine MEG, of whom nine could not be assessed, mainly due to artefacts caused by metals such as those of dental braces and fillings which were not mentioned during enrolment. Routine MEG showed a sensitivity and specificity of 31.6% and 78.4%, respectively, with a positive and negative predictive value of 90.9% and 32.2%, respectively (table 3).
No difference in sensitivity (p = 1.00) was identified between routine MEG and EEG as sensitivity for either was 31.6%. Sixteen true positives were identified on routine MEG that were not captured on routine EEG, which led to a diagnostic gain of 16.8%. Given the 100% specificity for routine EEG, a statistically significant difference in specificity (p = 0.008) was found in favour of routine EEG over routine MEG and no new true negatives were identified on routine MEG. The same analysis was performed for routine EEG in combination with additional EEG(s) and compared to routine MEG. A significant difference was found for both sensitivity (p = 0.002) and specificity (p = 0.039) in favour of combined routine EEG plus additional EEG over routine MEG. Of note, nine true positives and one true negative were identified on routine MEG, but not on routine and additional EEG, leading to a diagnostic gain of 9.5% for routine MEG.
Secondary outcome
In 19 patients, MRI showed a lesion that could cause the epileptic seizures. The location of the lesion was congruent with the localization based on the final clinical diagnosis in 73.7% and incongruent in 15.8%. In the remaining 10.5%, the final clinical diagnosis was uncertain, so no conclusion could be drawn regarding concordance. Of the 14 patients with congruent localization, routine MEG showed epileptiform discharges in five patients, no epileptiform abnormalities in seven, and two MEG recordings were not assessable. Localization of the epileptiform discharges on MEG were congruent with localization based on MRI and the final clinical diagnosis in all five patients, which corresponds to 35.7% of all patients with congruence between MRI and semiology.
Discussion
For patients referred for routine EEG as part of the diagnostic process for epilepsy, routine MEG demonstrated a sensitivity and specificity of 31.6% and 78.4%, respectively. Whereas routine EEG demonstrated equal sensitivity to that of MEG and specificity of 100%, routine EEG plus additional EEG showed a sensitivity of 52.6% and specificity of 97.3%. However, routine MEG showed true positives which were not identified on EEG. Diagnostic gains of 16.8% and 9.5% were reported for routine MEG compared to routine EEG and routine EEG plus additional EEG, respectively.
Remarkably, in 79.0% of the patients (n = 109), an additional EEG was performed, which increased the sensitivity from 31.6% to 52.6%. This could be explained by the fact that 24 of 32 patients, categorized as “epileptiform” on routine EEG, underwent an additional examination for further categorization of their epilepsy. Furthermore, it is noticeable that nine patients were diagnosed with epilepsy although routine EEG did not show abnormalities and no additional EEG was performed. In two of these patients, routine MEG was categorized as “epileptiform” and in another patient, MRI showed a possible epileptogenic lesion. For the true positives captured with MEG but not routine EEG, it is striking that 12 of 16 patients (75.0%) were diagnosed with focal epilepsy, three (18.7%) with generalized epilepsy, and in one patient (6.3%) the syndrome diagnosis was uncertain. For the true positives captured with MEG but not routine EEG plus additional EEG, approximately the same percentage of patients (77.8%) suffered from focal epilepsy. The other two of the nine patients (22.2%) were diagnosed with generalized epilepsy. Based on the number of patients with positive focal epilepsy on MEG with either routine EEG (50.0%) or routine EEG plus additional EEG (57.1%), MEG would appear to be particularly accurate in detecting focal epilepsy.
Previous studies have reported comparable sensitivity for routine EEG. A systematic review and meta-analysis reported a pooled sensitivity of 44.5% and specificity of 79.5% (Bouma et al., 2016). The pooled sensitivity in adult studies was significantly lower (p = 0.02) compared to children studies with a sensitivity of 17.3% and 57.8%, respectively. The sensitivity of this cohort (31.6%) can be explained by inclusion of patients aged between six and 74 years old. In the study of Keezer et al. (2016), based on a study population similar to ours, diagnostic accuracy for routine EEG was reported with a sensitivity of 26.0% and specificity of 100% (Keezer et al., 2016).
In a recent review, the main indications for long-term EEG, including the modalities of sleep-deprived EEG, 24-hour ambulatory EEG, and continuous prolonged video-EEG, were described (Michel et al., 2015). Furthermore, analysis of the diagnostic contribution of sleep-deprived EEG was described in the following three studies based in tertiary centres. Liporace et al. (1998) recorded interictal activity on EEGsd in 24% of the patients suspected of having epilepsy, in whom routine EEG was normal or non-diagnostic. Twenty-four-hour ambulatory EEG showed a similar increase in interictal activity and demonstrated a higher detection rate of epileptic seizures. In the retrospective study of Heers et al. (2010), the authors reported the occurrence of epileptic spikes on EEGsd in 51% of patients with focal and generalized epilepsy. Moreover, epileptic interictal abnormalities were reported in 41.2% patients on EEGsd following normal or non-specific routine EEG with a specificity of 91.1% in the study of Giorgi et al. (2013). Although these figures cannot be directly compared with ours due to differences in study design and populations, the observed (additional) diagnostic contribution of EEGsd, in comparison with routine EEG only, is in line with the above-mentioned studies.
A limited number of studies have been performed to evaluate the diagnostic accuracy of MEG, as it is mainly used for presurgical candidates. Duez et al. (2016) found a sensitivity of 41% for simultaneous MEG-EEG after repeat routine EEGs without abnormalities. Epileptiform abnormalities were detected by MEG during simultaneous recording in eight of 22 patients with epilepsy, of whom half were positive based on MEG only. This led to an increased sensitivity of 18% for MEG. Reported sensitivity and diagnostic gain for MEG in our cohort were comparable, although it is important to mention the difference in study design. The Denmark trial included patients with consistently normal EEGs. Whereas our study may be a better representation of routine care in a tertiary epilepsy centre, the more pronounced measures regarding the diagnostic accuracy for MEG in the study of Duez et al. may reflect the inclusion of patients who were difficult to diagnose. However, EEG and MEG have different sensitivity profiles (Lopes da Silva, 2010; Hunold et al., 2016), thus multiple negative EEG recordings do not necessarily forecast negative MEG recordings.
This study was performed according to the STARD criteria. However, limitations should be considered. According to our power analysis, the study was underpowered. The power analysis was performed on the assumption that MEG would detect more patients with epileptiform abnormalities compared to additional EEG, however, the major contribution of MEG seems to be identifying epileptiform abnormalities which are not detected by EEG. As it is not possible to obtain further information due to practical constraints, we decided to continue the study without an additional sensitivity analysis based on further assumptions. Furthermore no MRI (report) was available for 13 patients, even though MRI was considered to be part of routine care. Of these patients, eight were diagnosed with epilepsy, four with generalized and four with focal epilepsy, and five did not suffer from epilepsy according to the final clinical diagnosis. The (syndrome) diagnosis was not considered to be uncertain in any of the patients, which could possibly be an explanation for the lack of MRI. In addition, the study population is challenging due to diagnostic difficulties and refractory symptoms in the referring hospitals, for which an expert opinion was needed. Future research in secondary centres may provide informative results on the diagnostic accuracy of routine MEG compared to EEG. Unfortunately, routine MEG is not yet widely used, and more experience and funding are required. Finally, the routine MEG results were available to the treating physicians specialized in epilepsy when the final clinical diagnosis was made. This may have led to a bias, however, the diagnostic process for the participants was already long and potentially postponing diagnosis and treatment was deemed unethical. In future studies, we recommend that all available data are evaluated to determine a final clinical diagnosis, except for MEG data provided by independent physicians, in order to prevent possible bias. In addition, future studies should also incorporate secondary outcomes such as patient experiences and cost-effectiveness.
Conclusion
MEG is a useful additional technique in the diagnosis of epilepsy, as diagnostic gains of 16.8% and 9.5% were reported for routine MEG compared to routine EEG and routine EEG plus additional EEG, respectively.
Supplementary data
Summary didactic slides are available on the www.epilepticdisorders.com website.
Acknowledgements and disclosures
This study is not registered in a public database as it was intended as a healthcare innovation project. The protocol can be requested at Kempenhaeghe. An innovation fund supported this study (grant number: 12-018, file 2324). We would like to thank research assistant Marion Savelkoul and EEG technicians, Leonie van den Heuvel and Sonja Vervoort, for their contribution.
None of the authors have any conflict of interest to declare.
![]() This work is licensed under a
Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License
This work is licensed under a
Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License