Epileptic Disorders
MENUSensitivity of long-term EEG monitoring as a second diagnostic step in the initial diagnosis of epilepsy Volume 23, issue 4, August 2021
The correct diagnosis of epilepsy following the new ILAE guidelines [1] remains a major clinical challenge. In particular, practical difficulties arise when specialists need to evaluate the recurrence risk after a single first-ever epileptic seizure in order to establish whether a patient has epilepsy or not [1].
In this context, electroencephalography (EEG) is useful to detect typical interictal patterns in support of epilepsy diagnosis. However, the sensitivity of a 20-minute standard-EEG (S-EEG) is limited. In adult patients with epilepsy, the rate of S-EEGs without epileptiform abnormality is in the range of 40 to 60% [2, 3].
Therefore, if the suspicion of epilepsy remains considerably high, neurologists frequently perform long-term EEG monitoring (L-EEG) as a next diagnostic step [4]. This procedure is widely regarded as the most sensitive and specific diagnostic method in epileptology [5].
While the sensitivity of L-EEG as a single method has already been subject to several studies [5-7], separate analyses of the additional diagnostic value of L-EEG after a normal or non-diagnostic S-EEG are scarce [8, 9]. This is critical because the sequence of S-EEG and L-EEG constitutes a standard procedure in epileptology [10].
In this study, we aimed at evaluating the sensitivity and negative predictive value of L-EEG in patients being assessed for epilepsy, who had already undergone at least one non-specific S-EEG.
As secondary outcomes, we analysed whether: (1) non-specific changes, such as focal slowing on S-EEG, are associated with epileptic changes on L-EEG; and (2) whether clinical parameters, such as frequency of presumptive seizures, antiepileptic drug (AED) intake and cerebral imaging, are associated with positive L-EEG findings or epilepsy diagnosis.
Material and methods
We retrospectively analysed the medical histories of patients (male and female, age > 16 years), who were clinically assessed at the University Hospital of Zurich and had undergone an L- EEG examination for at least 48 hours at the University Hospital Zurich or the Swiss Epilepsy Center Zurich. All of them underwent at least one S-EEG without epileptiform abnormalities from 2010 to 2015. We included patients with both inpatient monitoring and ambulatory L-EEGs.
Other inclusion criteria were:
- •full documentation of patient history, EEGs and diagnosis ;
- •detailed witness accounts of the seizures ;
- •no diagnosis of epilepsy made before L-EEG ;
- •no specific epileptiform abnormalities on S-EEG before L-EEG ;
- •documented cerebral imaging via magnetic resonance imaging (MRI) or computer tomography (CT).
The exclusion criteria were:
- •patients who declined the use of their data ;
- •no adequate documentation of the anticonvulsive medication taken.
Out of the 75 patients included in our study, 10 patients (13%) received antiepileptic treatment during the time of the recordings. The proportion of treated patients did not differ between the epileptic and non-epileptic group (table 1). The treating physicians did not reduce or stop AEDs before or during L-EEG.
Standard and long-term EEG study procedure
All patients underwent 23-channel surface L-EEG. The 10-20 system and T1 and T2, as extra electrodes, was used for all recordings. Cardiac monitoring was provided through a one-channel electrocardiogram. The duration of L-EEG was always longer than 48 hours. Natus cup electrodes fixed with EC2®-electrode cream and secured by a hair net were used for the L-EEG recordings. An XLTEK® EEG recording system with a sampling rate of 500 Hz acquired the data. L-EEG data were stored in and retrieved from a Natus NeuroWorks software database. We evaluated L-EEG traces through bipolar longitudinal and transverse montages, as well as through average reference. All inpatients also underwent inpatient video monitoring. Patients were provided with an event button and a diary in order to document seizure events. Inpatient L-EEGs were performed at the general neurology ward and patients were thus clinically monitored by the medical personnel of the ward.
The S-EEGs were conducted over a period of at least 20 minutes, in compliance with the requirements of the German Society for Clinical Neurophysiology (DGKN, 2006) [11], using silver-plated Ag/AgCl-electrodes and a Nihon Kohden EEG-1100 EEG. As for L-EEG, the 10-20 system and T1 and T2 as extra electrodes, as well as single-channel ECG, was used for all recordings. We reviewed all S-EEG data using Megis EEG Focus software.
L-EEGs in general were labelled “positive” if they revealed interictal epileptiform activity (i.e. spike-wave activity, polyspike-wave activity, sharp waves and spikes) in a focal or generalized distribution or epileptic sequences. To define epileptiform vs. non-epileptiform abnormalities, we relied on the criteria provided by Gloor [12] for diagnosing epileptiform potentials. Other variants, such as general or focal slowing or patterns of uncertain significance, were described as non-specific and we consequently labelled the L-EEG as “negative”.
Epilepsy diagnosis
The study investigators made the clinical diagnosis of epilepsy in accordance with ILAE guidelines [1]. To this end, the patient histories were thoroughly evaluated for each case, and a diagnosis of epilepsy was made based on any of the following:
- –at least two unprovoked definite epileptic seizures, 24 hours apart, as documented by detailed and reliable witness records;
- –at least one definite epileptic seizure and a recurrence risk higher than 60% [13] for a second seizure. A high recurrence risk is indicated by the presence of IEDs on L-EEG or by a typically epileptogenic lesion on brain MRI, fitting with seizure semiology [1].
Therefore, in the setting of our study, a positive L-EEG led to the immediate diagnosis of epilepsy. In the case of non-specific L-EEGs, epilepsy diagnosis was made when at least one other clinically definite epileptic seizure was documented after L-EEG or typical epileptogenic lesions, fitting with seizure semiology, were reported. In this case, we deemed the L-EEG as “false-negative”.
In short, the clinical diagnosis of epilepsy was the benchmark against which we compared the sensitivity of L-EEG.
Psychogenic seizures were either diagnosed via L-EEG or observation during follow-up EEGs. Syncope was diagnosed based on suggestive findings in the cardiac diagnostic workup (including Holter monitor, echocardiography and tilt-table test). All other diagnoses listed and described above -due to their very nature- were diagnosed through thorough history taking concerning semiology of the events, in combination with known comorbidities.
Statistical analysis
For each patient, we recorded the following parameters:
- •occurrence of interictal epileptiform discharges (IEDs) on S-EEG, sleep-deprived EEG and L-EEG
- •total frequency of seizures
- •occurrence of events suspected to be seizures during the recordings
- •neuroimaging findings
- •antiepileptic medication taken
Statistical analysis was carried out, where appropriate, using SPSS 23.0 software by IBM. Fisher and Mann-Whitney tests were generally used to assess significance (tables 1, 2).
Results
Demographic and clinical characteristics of the study population
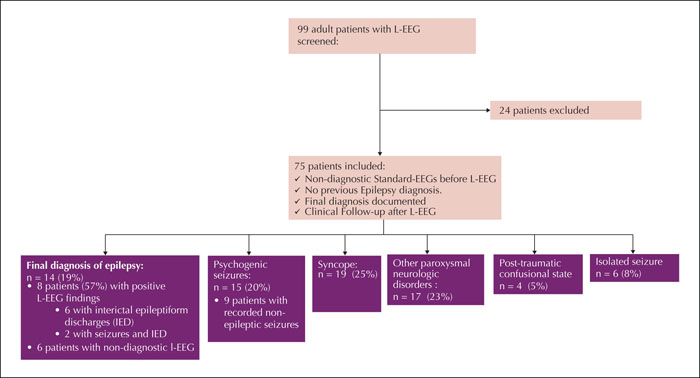
We analysed the medical histories of 99 patients, who had obtained an L-EEG as second-line diagnostic after S-EEG during the time span from 2010 to 2015. Out of this group, 75 patients (mean age: 42±18.3 years; 33 [43%] females) fulfilled the inclusion criteria for the study (figure 1). Sixty-nine (92%) patients underwent inpatient long-term monitoring, while six (8%) underwent ambulatory L-EEG. The average length of the recordings was 71.1±15.8 hours (range: 48-168 hours), with most of the recordings lasting between 70 and 75 hours (62 patients; 82.7%). Reasons for undergoing S-EEG and L-EEG were unexplained loss of consciousness in 33 patients (44%), episodes of altered consciousness in 15 patients (20%), a first generalized tonic-clonic seizure in 13 patients (17%), or episodes of unexplained transient focal neurological symptoms in 14 patients (19%).
Fourteen (19%) of the patients were finally diagnosed with epilepsy.
Among the non-epileptic diagnoses, syncope (either vasovagal or cardiogenic) was the most frequent (n=19, 25%) (figure 1). Fifteen patients (20%) were diagnosed with psychogenic seizures. Seventeen (23%) patients had other paroxysmal neurological disorders (migraine aura, cataplexy). Six patients were diagnosed with an isolated or early-provoked epileptic seizure. In the remaining four patients, the treating physicians interpreted the respective episode of confusion as the result (but not cause) of traumatic brain injury, i.e. they probably never had epileptic seizures but their episodes of confusion after brain injury were mistaken for focal seizures.
In accordance with the inclusion criteria, all patients underwent at least one S-EEG before the L-EEG. About one third of the patients (n=26, 34.6%) obtained two or more S-EEGs before the L-EEG with a range of two to seven EEGs in one case (1.6±1.1 EEGs on average).
Every patient underwent cerebral imaging (either MRI [n=71], or CT [n=4]).
In 16 patients (21.3%), neuroimaging showed potentially epileptogenic lesions, e.g. cortical malformations or lesions, but in only in three cases, these changes were deemed to be specific and “fitting with seizure semiology”, as stated by Fisher et al. [1]. Therefore these findings were not significantly associated with a positive epilepsy diagnosis (28.6% in epileptic vs. 19.6% in non-epileptic patients, p<0.48).
The proportion of patients who reported having had more than two seizures was smaller in the epilepsy group than in in the non-epileptic group (42.9% vs. 72.1%), though this finding was not statistically significant (p<0.057).
Sensitivity and negative predictive value of L-EEG and association between IEDs and other findings
Out of the 14 patients finally diagnosed with epilepsy, eight showed either IEDs alone (n=6) or both IEDs and ictal patterns (n=2) on the L-EEG, leading to a positive classification. Two of the eight patients showed IEDs only during sleep while five showed them during wakefulness as well as sleep, and one patient only expressed IEDs during wakefulness. None of the 61 non-epileptic patients exhibited IEDs on L-EEG. This corresponds to a negative predictive value of 91% for L-EEG, with a significantly lower sensitivity of 57%.
The appearance of IEDs on L-EEG was not associated with other pathological changes on L-EEG, such as focal or general slowing (table 2).
Only the two patients in the epilepsy group who showed ictal patterns (14%) also reported having had seizures during their ambulatory L-EEG recordings – in both cases, focal seizures. In contrast, 16 (26%) of the non-epileptic patients stated that they had had seizure events during their recordings (p<0.50) (table 1). Through combined video and EEG monitoring, we documented psychogenic non-epileptic seizures in nine of these patients, leading to the diagnosis of psychogenic seizures. Thus, 60% of all patients finally diagnosed with psychogenic seizures were diagnosed based on L-EEG (figure 1). In the remaining seven patients, no specific abnormality was confirmed during the self-reported event – neither through EEG nor through video-monitoring.
As there were only very few patients with ambulatory L-EEG (n=6), we refrained from a comparative analysis between in-hospital L-EEG and ambulatory L-EEG. The sensitivity and negative predictive value calculated for the in-hospital L-EEG subgroup (58% and 92% respectively) did not differ significantly from that for the overall cohort (see above).
Only one patient with IEDs showed an abnormality on MRI, namely bilateral periventricular heterotopy.
Other EEG findings
Due to the inclusion criteria, none of the S-EEGs revealed IEDs or ictal patterns. Nonetheless, 30.6% of all patients exhibited focal slowing on S-EEG. General slowing was present in only 2.7% (n=2) of the patients.Out of the patients diagnosed with epilepsy, seven (50%) had focal slowing on L-EEG, while 21 (34.4%) of the non-epileptic patients showed focal slowing. Therefore focal slowing was neither significantly associated with the epilepsy diagnosis itself (p<0.36) (table 1) nor with the appearance of IEDs (p<0.46) (table 2).
Seven patients (9.3%) exhibited patterns of unknown significance, among them wicket spikes (n=4), small sharp spikes (BETS, n=1) and steep transients (n=1). Due to the small number and heterogeneity of these patterns, we refrained from further statistical analysis concerning these subgroups.
Discussion
In this retrospective study, we examined the sensitivity and negative predictive value of L-EEG monitoring as a second diagnostic step after a negative S-EEG. To this end, we selected and analysed the EEGs and clinical data of 75 patients who were evaluated for epilepsy. Several studies have addressed the diagnostic value of L-EEG either as a single method [6, 7] or also in comparison to S-EEG [3, 14]. Nonetheless, L-EEG as a second diagnostic step after a non-diagnostic S-EEG has so far not been the primary scope of a study. We chose this specific study cohort, because this sequence of diagnostic work-up is a frequently chosen approach in epileptology [10].
Upon examination of the data of 75 patients, we provide evidence that L-EEG is also a reliable diagnostic method in this preselected group. In particular, the method features a high negative predictive value of 91%. On the other hand, due to a significant false negative rate (43%), the sensitivity is limited. Therefore L-EEG appears to be most useful at excluding epilepsy based on this study.
Concerning sensitivity, recent studies on ambulatory long-term monitoring [3, 7, 14] reported values ranging from 38% [13] to 68% [7]. However, these numbers refer to sensitivity relating to all possible seizure aetiologies (i.e. including non-epileptic seizures, syncope, etc.), while our study focused solely on epilepsy diagnosis.
Due to the study design, the reason(s) for this significant false negative rate (43%) could not be determined. One obvious explanation could be the limited capability of surface EEG in detecting pathological discharges generated in deep-lying structures (e.g. the orbitofrontal or the temporomesial cortex) and its low spatial resolution [15].
We also focused on the association between positive L-EEGs and other pathological EEG findings. Interestingly, focal slowing was neither associated with epilepsy diagnosis nor the appearance of IEDs on L-EEG in our study. This finding is in contrast to some other L-EEG studies [5, 14] showing that epilepsy diagnosis was confirmed in patients who already had focal changes on a previous EEG. While we have no final explanation for this difference in observations, we assume that this could be due to an effect of preselection. Focal slowing is quite common and a very non-specific pathologic finding [16], however, patients showing (non-specific) focal changes on S-EEG were in turn more likely to be chosen for an L-EEG.
We also found no significant association between AED intake and positive/negative L-EEG results. It is still a matter of debate as to whether AEDs reduce the occurrence of IEDs [17] or whether they show no influence [18]. However, the examined subgroups are probably too small to single out a potential small effect of AEDs.
Surprisingly, the study data showed no positive association between the number of reported seizures and epilepsy diagnosis or appearance of IEDs. In fact, the proportion of patients reporting more than two episodes was higher in the non-epileptic group. This observation could be due to the fact that patients suffering from psychogenic non-epileptic seizures (PNES) were often referred with a long history of seizures while patients with (oligo-) epilepsy were mostly evaluated for the first time.
There are several limitations to this study. Firstly and most importantly, as L-EEG is deemed the diagnostic golden standard, there is no effective control for eventual positive results. Therefore, the appearance of IEDs on L-EEG almost invariably leads to a positive epilepsy diagnosis. Consequently, we refrain from statements concerning specificity and positive predictive value for this diagnostic method. Secondly, this was a retrospective study. We acknowledge that the lack of a clear-cut algorithm based on symptom constellation is a limitation of this study. The clinical decision for an L-EEG was not standardized and therefore prone to selection bias, e.g. in clinical practice, patients presenting with an atypical/dubious seizure semiology are more likely to undergo an L-EEG. This led to a relative small proportion of patients who were finally diagnosed with epilepsy. On the other hand, this study cohort underlines the high potential of L-EEG in discriminating between epileptic and non-epileptic disorders.
As the study was focused on sensitivity of L-EEG after non-diagnostic S-EEG, no direct comparison was possible between the sensitivity of a single (or repeated) S-EEG and L-EEG in detecting epileptiform changes.
Though the criteria for diagnosing IEDs by Gloor [12] were strictly abided by the examiners, the differentiation between epileptiform and non-epileptiform patterns on EEG is often not clear-cut and relies on the experience of the respective epileptologist.
Another potential limitation to our study is the different length of L-EEG recordings in our study; in the study of Faulkner et al., [7] 96% of all IEDs were reported to occur within the first 48 hours of reforming.
The inclusion of both patients taking AEDs and patients without AEDs may also pose a limitation to this study. Nonetheless, due to the small size of the AED subgroup (n= 10) and the fact that two of these patients actually had a positive L-EEG, we do not believe that AED intake played a significant role as a confounding factor in this study. As a general problem, the diagnosis of epilepsy is partly based on the report of witnesses and the patient him/herself. Though we tried to minimize this through structured interviews in our consultations, the final diagnosis always relied on the subjective judgment of a neurologist.
As a whole, the study shows that in this preselected cohort, L-EEG is a reliable method with a high negative predictive value but with limited sensitivity. Therefore, a negative L-EEG does not exclude epilepsy and thorough evaluation of seizure history and clinical findings remains crucial for a reliable diagnosis.
Supplementary material
Summary slides accompanying the manuscript are available at www.epilepticdisorders.com.
Disclosures
Authors did not declare their potential conflicts of interest.