Epileptic Disorders
MENUSelective deep brain stimulation in the substantia nigra reduces myoclonus in progressive myoclonic epilepsy: a novel observation and short review of the literature Volume 21, issue 3, June 2019
Progressive myoclonic epilepsy (PME) includes a variety of phenotypically similar, but genetically heterogeneous syndromes that manifest with common clinical features such as progressive symptomatic myoclonus (including both action-induced positive and negative myoclonus), generalized epileptic seizures, ataxia, and cognitive impairment. Both predominant symptoms (epileptic seizures and myoclonus) are often pharmacotherapy-resistant (Minassian et al., 2016; Genton et al., 2016). Due to the frequent association with ataxia and cognitive impairment, the progression of myoclonic jerks in PME leads to significant impairment of quality of life and eventually loss of the patient's autonomy.
Common underlying diseases causing the clinical phenotype of PME are Lafora's disease, Unverricht-Lundborg disease, Gaucher disease, and myoclonic epilepsy with ragged red fibers (MERRF) (Shahwan et al., 2005).
Antiepileptic drugs such as benzodiazepines, valproic acid, zonisamide, barbiturates, chloral hydrate, topiramate, levetiracetam, and piracetam are most commonly used for the treatment of epileptic seizures in PME (Genton and Guerrini, 1990; Pranzatelli and Tate, 2001; Uthman and Reichl, 2002; Conry, 2004; Crest et al., 2004; Aykutlu et al., 2005; Mancuso et al., 2006; Vossler et al., 2008). Myoclonus can be treated with valproic acid, levetiracetam, and clobazam, although the efficacy of these substances is usually limited.
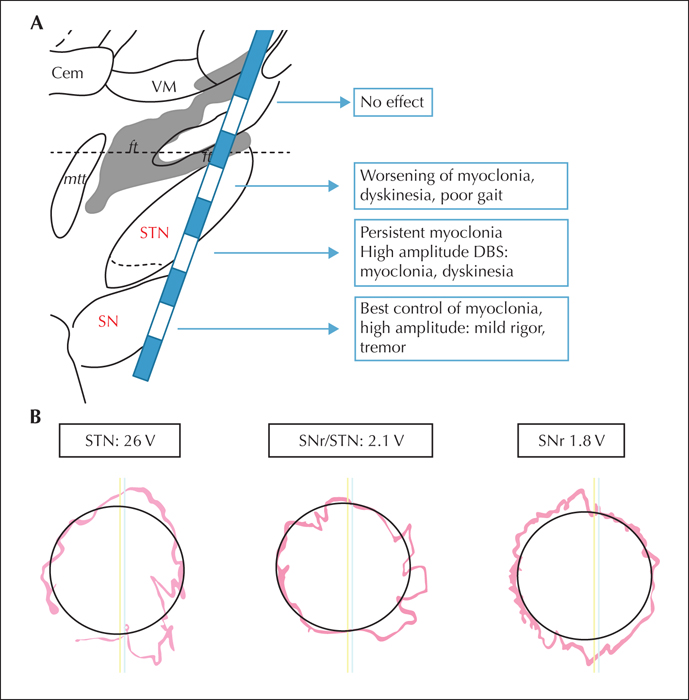
High-frequency deep brain stimulation (DBS) of the transitional zone between the subthalamic nucleus (STN) and the substantia nigra pars reticulata (SNr) has provided promising results in the treatment of patients with PME (Vesper et al., 2007; Wille et al., 2011). Wille and co-workers reported sustained partial or complete reduction in myoclonus and seizure frequency after STN/SNr DBS (table 1). Other groups reported DBS in the STN to be effective for seizure reduction in epilepsy patients (Chabardès et al., 2002; Benabid et al., 2002) (table 1). As summarized in table 1, the stimulation of STN or the SNr/STN transitional zone shows a beneficial effect on either seizure frequency or myoclonus. However, the effect of selective stimulation of the SNr has not been investigated so far.
Case study
A male patient with the clinical phenotype of PME was referred to our centre for evaluation of DBS at the age of 32. At the age of nine years, he had developed tremor and progressive rest-, action- and reflex myoclonus with postmyoclonic atony. Two years later, the patient suffered from several bilateral tonic-clonic seizures and anticonvulsive therapy with valproic acid was initiated. Due to worsening of tremor, myoclonus, and generalized epileptic seizures within the next years, the anticonvulsive treatment was changed several times (including valproic acid, sultiame, piracetam, levetiracetam, ethosuximide, zonisamide, and clonazepam). Bilateral tonic-clonic seizures were well controlled under treatment with valproic acid, levetiracetam, zonisamide, clonazepam, and piracetam.
Unverricht-Lundborg/EPM1 was excluded by molecular genetic testing. No relevant mutations were found upon mitochondrial genome testing associated with MELAS, MERRF or Leber's hereditary optic neuropathy. MR imaging revealed bilateral optic nerve hypoplasia, intraventricular septa, and subcortical cysts, as a possible indication for congenital or neonatal CMV infection.
In the further course of the disease, the patient showed progressive pharmacotherapy-resistant disabling myoclonus, tremor, dysarthria, and rare epileptic and non-epileptic seizures (generalized tonic-clonic seizures occurred with a frequency of one per six months), as well as progressive gait disorder of mixed aetiology (ataxia and visual impairment and myoclonus), all of which were resistant to pharmacotherapy.
Based on a few encouraging reports demonstrating improvement of myoclonus after DBS in PME (Vesper et al., 2007; Wille et al., 2011), we discussed this treatment option with the patient. However, as previous reports (Benabid et al., 2002; Chabardès et al., 2002) showed significant reduction in epileptic seizure frequency also upon stimulation of the subthalamic nucleus, we decided to implant the electrode along a trajectory, allowing postoperative selective stimulation of either the STN, the SNr or the border zone in between these targets (figure 1A). In March 2015, the electrode implantation was performed using MR-based direct targeting and stereotactic intraoperative CT (Medtronic Leads 3389). Planning coordinates relative to the midcommissural point were: x= + 11 mm, y= -4.0 mm, and z = -6.0 mm. Electrode position was verified by postoperative CT scan and reconstruction of electrode positions.
Post-operative assessment and stimulation testing
The primary focus for optimization of stimulation was a reduction in myoclonus and improvement in gait and balance. Initially, the patient was treated with bilateral monopolar stimulation in the border zone between the STN and SNr, as in the previously published cases (Vesper et al., 2007; Wille et al., 2011). This strategy produced some improvement of myoclonus, mobilisation, speech, and mobility. Furthermore, in the first six months after implantation, the patient remained seizure-free with respect to generalized tonic-clonic epileptic seizures. Regarding myoclonus, the overall stimulation effect was limited and increasing stimulation amplitude up to 5 V induced an increase in myoclonus. We therefore performed an assessment of selective stimulation in a controlled setting, testing all approachable targets separately (figure 1A).
Upon stimulation in the STN/SNr, we observed mild dysarthria, psycho-motor slowing, but improvement in myoclonus of the arms and legs as compared to before DBS. At that time, monopolar stimulation in the STN/SNr transitional zone, with 2.1 V, pulse width of 90 μs, and frequency of 130 Hz, was applied. After increasing the monopolar stimulation to 2.6 V in the STN/SNr transitional zone, we observed worsening of gait function, myoclonus, and fine motor skills (figure 1B).
Next, we tested monopolar stimulation of the motor STN with identical stimulation parameters (2.6 V), but this procedure provoked severe worsening of rest- and action-myoclonus and generalized dyskinesia (figure 1B).
Finally, we applied monopolar stimulation to the SNr (1.8 V) with immediate positive effects on myoclonus and amelioration of gait function and fine motor skills (figure 1B). Furthermore, we observed clinically relevant improvement in gait and hand motor control with an increase in monopolar stimulation of the SNr up to a stimulation amplitude of 2.5 V. Upon further increase of the SNr monopolar stimulation up to 4 V, we observed mild Parkinsonism with rest tremor of both hands, accompanied by mild rigor of the extremities, and the patient reported a light sense of stiffness in the trunk, all of which were reversible upon reduction of the stimulation amplitude.
These responses were easily reproducible and further documented by videography, accelerometers, and standardized motor performance testing (figure 1B). The assessment of myoclonus and gait function based on blinded video analysis is outlined in supplementary table 1. After repeated comparative testing of stimulation, we decided to program monopolar stimulation of the SNr which later on produced persisting beneficial effects that remained ever since, i.e. more than 24 months post operation. The patient also remained seizure-free for bilateral tonic-clonic seizures.
Discussion
In line with previously published case series showing beneficial effects in adult patients with PME undergoing DBS (Benabid et al., 2002; Chabardès et al., 2002; Vesper et al., 2007; Wille et al., 2011), we present a further case with effective long-term effect of DBS in PME. However, in contrast to the previous reports with high-frequency DBS applied in the transitional zone between STN and SNr (Vesper et al., 2007; Wille et al., 2011), our patient showed the most significant effect upon selective monopolar stimulation of the substantia nigra. This conclusion was derived from a selective comparative stimulation of three target regions (as described above) identifying SNr as the most effective in suppression of myoclonus.
In the border zone between STN and SNr, we observed similar results as reported by Wille and Vesper (Vesper et al., 2007; Wille et al., 2011), however, the beneficial effect was limited to lower voltage, whereas high-voltage DBS lead to worsening of symptoms, similar to those observed in monopolar STN stimulation (supplementary table 1). Therefore, the detrimental effect could be explained by a concomitant stimulation of the STN at higher amplitude. In this line, we observed hyperkinesia and worsening of myoclonus upon selective stimulation of the motor subthalamic region. This observation fits well within the concept of subthalamic DBS in the STN for movement disorders: STN stimulation ameliorates bradykinesia in Parkinson's disease. Therefore, it is possible that STN DBS results in dyskinesia in a patient not suffering from Parkinsonism. As myoclonus is a hyperkinetic movement disorder, STN stimulation consecutively leads to worsening of symptoms, rather than having a therapeutic effect. In a similar vein, we know from Parkinson patients, that DBS of the SNr has an anti-kinetic effect1.Therefore, the reduction in myoclonus by induction of mild Parkinsonism is clearly explained by the effect of DBS within the basal ganglia model of hypokinetic movement disorders (Krauss and Volkmann, 2004). Alternatively, considering the pathophysiology of PME with cortical and subcortical hyper-excitability, the positive effect of SNr stimulation might be based on a disruption of the pathological hyperactive cortico-subcortical pathways.
Although this single case does not allow for a general conclusion on target selection in PME, our findings encourage an implantation trajectory that postoperatively allows for selective SNr stimulation, as this target region was the most effective in suppression of myoclonus. On the other hand, regarding the positive effect on epileptic seizures in the literature, in patients with predominant epileptic seizures, interleaved stimulation of both the STN and the SNr might be beneficial.
In conclusion, we suggest implanting at least one electrode contact in the substantia nigra pars reticulata in patients undergoing DBS for PME to allow postoperative selective monopolar stimulation of this target. A pragmatic postoperative approach might be to increase DBS amplitude in the SNr until the observation of mild Parkinsonism and then reduce the amplitude until only the beneficial effects persist.
Supplementary data
Supplementary table is available on the www.epilepticdisorders.com website.
Disclosures
None of the authors have any conflict of interest to declare.
1 Occasional intraoperative stimulation of the SNr in Parkinson's patients includes acute bradykinesia.