Epileptic Disorders
MENUSeizure-induced reversible MRI abnormalities in patients with single seizures: a systematic review Volume 23, issue 4, August 2021
Seizures are a common clinical presentation, with up to 10% of the population in developed countries experiencing a seizure in the course of their lifetime [1]. According to the 2010 Global Burden of Disease Study by the WHO, epilepsy accounts for a quarter of the global neurological burden with regards to disability-adjusted life years [2]. The use of magnetic resonance imaging (MRI) has become increasingly common in the investigation of seizure patients, and several researchers have reported seizure-induced reversible MRI abnormalities (SRMA) [3, 4].
The pathophysiology of seizure-induced MRI changes is incompletely understood. The abnormalities have been variably postulated to be related to cytotoxic oedema, vasogenic oedema, and increased energy metabolism resulting from the ictal episode [5, 6].
MRI abnormalities detected following a seizure can be due to reversible seizure-induced changes, the underlying cause of the seizure (e.g. tumour), or unrelated incidental findings. It can be difficult to differentiate seizure-related MRI abnormalities (SRMA), which are caused by the seizure activity per se, from underlying pathologies that require specific management, for example, infection, inflammation, tumours, and infarcts [7]. This can make it challenging to reconcile post-ictal neurological deficits with abnormalities seen on MRI. Against this backdrop, we undertook this systematic review to define the characteristic features of SRMA amongst patients who have suffered a single seizure, based on localisation and seizure types. Typical findings on commonly used MRI sequences and time for abnormalities to resolve were investigated. We hope that the findings will aid the differentiation of SRMA from other pathologies, and assist decision-making by clinicians.
Methods
Eligibility criteria
The inclusion criteria we used were as follows: primary research articles with:
- •investigation of reversible seizure-induced MRI abnormalities;
- •patients presenting with single unprovoked seizures;
- •availability of descriptive MRI results after a seizure, as well as follow-up;
- •and complete resolution of MRI abnormality at follow-up.
We excluded publications based on status epilepticus, cluster seizures, partial resolution of MRI abnormalities at follow-up, provoked seizures including febrile seizures, grey literature, animal studies, non-English literature, and review publications. In order to maximise the data spread, we did not set an age limit. The inclusion and exclusion criteria were applied to each individual case reported in the publications. The list of articles was finalised in May 2020.
Search strategy
We performed a comprehensive literature search of the electronic databases PubMed (1996 to April 2020), Medline (1946 to April 2020), and Embase (1947 to April 2020). The key search terms included synonyms and variations of the words “seizure”, “magnetic resonance imaging”, “reversible”, “induced” and “abnormality”. We combined the search terms with Boolean operators. Details of the search strategy are included in supplementary table 1. The search strategy was developed by two investigators (FM and US) with the help of a medical librarian and the search was conducted by the first author (FM). Two authors (FM and SM) independently screened the titles and abstracts in order to determine eligibility of relevant articles, followed by a review of full texts using “Covidence”, a web-based platform for systematic reviews (www.covidence.org). Any discrepancy between the two authors was adjudicated by a third investigator (US). The reference lists of the selected papers were then manually screened by two investigators (FM and SM) independently, with discrepancies adjudicated by another (US), for additional suitable articles that were not detected in the primary database search. We conducted this systematic review according to the Preferred Items Reporting for Systematic Review and Meta-Analyses (PRISMA) guidelines [8].
Data extraction
Using Microsoft Excel, a data extraction form was prepared to record relevant data (supplementary table 2). The first author (FM) extracted the data which were subsequently cross-checked by another investigator (SM). Additionally, all imaging data in the spreadsheet were reviewed and crosschecked against the source publication by a neuroradiologist (SA). Discrepancies in data were discussed and finally adjudicated by another investigator (US). Details collected included age and sex of patients, country of study, epilepsy classification, seizure classification, duration of epilepsy diagnosis, duration of index seizure, associated symptoms, seizure focus, aetiology, treatment at presentation, anti-seizure medications (ASMs), seizure frequency, EEG features, the interval between index seizure and initial MRI, the interval between index seizure and follow-up MRI, and MRI data. We collated information related to MRI such as the abnormal MRI sequence (diffusion weighted imaging [DWI], apparent diffusion coefficient [ADC], fluid-attenuated inversion recovery [FLAIR], T2, T1, post-contrast), region, laterality (unilateral and bilateral), and anatomical localisation (cortical, subcortical, deep grey matter). Unreported or unspecified data were recorded as “not reported” or “unknown”. We classified epilepsy as either focal or generalised. First unprovoked seizures were grouped as a separate category. Seizures were classified according to the International League against Epilepsy (ILAE) operational classification of seizures [9]. Supplementary table 2 contains detailed and final data extracted from all included publications.
Quality assessment
Given the uncontrolled study design, we used a tool based on an eight-item questionnaire [10] in order to conduct bias and quality assessment. Due to variability in data quality within the cases of each paper, we assessed each case separately using this questionnaire. The papers were assessed according to the domains of selection, exposure to single seizures, the outcome of SRMA, follow-up, and reporting. Each of these domains was scored as either a “yes” or a “no”.
Synthesis of results
We synthesized the data qualitatively utilising tabular and graphical forms. Descriptive statistics, including frequencies of discrete variables and the mean/median of continuous variables, were reported.
Results
Study and population characteristics
Clinical features and demographics of all included subjects are summarized in table 1. Our search and screening process yielded a total of 11 publications [11-21]. In total, these 11 publications described 66 cases. Of these, 27 patients fulfilled the eligibility criteria. The median age of these patients was 24 years (range: 4 to 62), 13 were male (48%), eight were female (30%) and the sex of six (22%) was not specified. Five of the subjects were from case reports and the remaining 22 were selected from case series. The year of publication ranged from 1992 to 2017. Concerning the type of epilepsy, four patients (15%) were diagnosed with generalised epilepsy, whilst nine patients (33%) presented with focal epilepsy. Fourteen cases (52%) presented with first unprovoked seizures.
Seizures were classified as follows: four patients (15%) had generalised tonic-clonic seizures (GTCS) of generalised onset; three patients (11%) had GTCS of unknown onset; eight patients (30%) presented with focal to bilateral tonic-clonic seizures (FBTCS); two patients (7%) reported focal aware seizures (FAS), and eight patients had focal impaired awareness seizures (FIAS). There was also one case each of focal unspecified and subclinical (electrographic) seizures.
In 24 cases (89%), the aetiology of the seizure was either not known or not reported. There were two patients in whom an occipital tumour resection cavity was the established aetiology and in one case, a frontal cortical lesion was reported. At the time of the presentation, three patients were treated with carbamazepine, and one received phenytoin. The initial treatment was not reported in the remaining cases. In terms of clinical outcomes, four patients were reported to be seizure-free for 12, 18, 24 and 28 months, respectively. A shorter period of seizure freedom (five weeks) was reported in another case. Two patients were reported to have ‘well-controlled’ seizures. For the remaining 20 cases (74%), clinical outcomes were not specified.
Bias and quality assessment
All 27 cases demonstrated acceptable selection and documentation of the radiologic outcome of SRMA. Follow-up was acceptable in 26 (96%) cases. However, there was considerable variation in the reporting quality in the cases, with only 18 cases (67%) scoring acceptably. The shortcomings in reporting were primarily related to the timing of MRI scans, SRMA data, and clinical outcomes. Supplementary table 3 provides further details regarding the bias and quality assessment of this study.
MRI abnormalities
MRI time intervals
The time interval between the abnormal scan and the index seizure was reported with considerable variability, with many publications only giving a time range. Six patients (22%) had brain MRI within one day of the index seizure, two patients (7%) within two days, and two patients within three days. Additionally, eight patients (30%) had brain MRI between two and seven days after the index seizure. In nine cases (33%), the time gap between brain MRI and the index seizure was not specified. The median time interval between the index seizure and follow-up brain MRI, demonstrating complete resolution of SRMA, was 39 days (range: 5 to 720 days). No studies reported whether serial MRI scans were conducted for investigation of SRMA.
MRI changes according to the interval from index seizure
Brain MRI changes according to the timing of the initial scan are summarised in table 2. Of the six cases that underwent brain MRI within one day of seizure, two showed hypointensity on T1-weighted images, three demonstrated hyperintensity on T2-weighted images, and three showed contrast enhancement. T2 hyperintensity and contrast enhancement were seen in both cases in which MRI was performed within two days. Two patients who had MRI within three days of the index seizure had T1 hypointensity and T2 hyperintensity. In eight cases, only a range of times (two to seven days) between brain MRI and index seizure was given. Seven of these patients had hyperintensity on T2-weighted images, and five displayed hyperintensities on diffusion-weighted imaging (DWI).
MRI sequence
Abnormalities according to the MRI sequence are summarized in table 3. T2-weighted imaging was the most frequently reported sequence with data available in 24 cases (89%). Of these, hyperintense signal change was reported in 21 cases (78%). A figure for follow-up brain MRI, demonstrating resolution of signal change, was included in only 12 cases (44%). In the remaining 15 cases (56%), complete resolution was reported but supporting images were not included.
Anatomical and Lobar distribution of SRMA
The anatomical and lobar distribution, including laterality of SRMA, is summarized in tables 4 and 5. The anatomical location of SRMA was derived from the data reported on all specified MRI sequences. Unilateral SRMA was found in two thirds of the cases, while bilateral SRMA was seen in the remaining third. With regard to the specific anatomical location, cortical and subcortical regions were involved frequently, with 12 (44%) patients demonstrating SRMA in the cortical layer and 11 (41%) in the subcortical layer.
SRMA according to seizure classification
MRI features according to seizure classification are summarised in table 6. When it came to laterality of SRMA, 16/20 (80%) of patients with focal seizures displayed unilateral abnormalities while 5/7 (71%) with generalised seizures displayed bilateral abnormalities. Of the 16 focal seizure cases that exhibited unilateral SRMA, the abnormality was ipsilateral to the seizure focus in all nine cases in which sufficient information on seizure focus was provided.
Composite patterns
We visually analysed the anatomical distribution of SRMA to identify stereotypical constellations of findings, which we labelled as “composite patterns”. These composite patterns are summarised in table 7.
The most common pattern we identified was prominent subcortical signal changes with or without cortical or leptomeningeal involvement in 11 patients (41%). Out of those 11 patients, the MR images were published in only four (Case 4 of [7], Case 1 of [11, 15, 21]). In all four cases, the main signal abnormality was seen in the juxtacortical region. Four cases (15%) displayed a predominant gyral (cortical) pattern with or without leptomeningeal involvement. SRMA in the splenium was noted in two cases (7%). Another frequently identified pattern was hippocampal involvement, bilateral or unilateral, in seven cases (26%).
Discussion
Summary of evidence
In this systematic review of 27 patients exhibiting SRMA, we found that signal abnormalities can appear as early as six hours and resolve as soon as five days following a single unprovoked seizure. The median time for the complete resolution of MRI abnormalities among the reported patients was 39 days. We observed four characteristic patterns of signal change involving predominantly cortex, subcortical white matter (in particular, juxtacortical region), the hippocampus, and splenium. Signal changes in thalami were reported in only one case. Generalised seizures typically presented with bilateral SRMA whilst focal seizures frequently exhibited unilateral changes ipsilateral to the seizure focus. Abnormalities were most frequently observed on T2-weighted sequences, and enhancement on post-contrast imaging was also a common finding.
Clinical implications
The composite patterns that we identified, as well as the timing of development and resolution of SRMA, are likely to be the most clinically useful findings of our study. When reviewing brain MRI of patients who could potentially have SRMA, it is important for clinicians to take into consideration the seizure focus and seizure classification, as well as the time elapsed since the seizure. All available MRI sequences (pre- and post-contrast, T1, T2, FLAIR, diffusion trace, and ADC) should be reviewed to maximize the yield. Further, serial imaging follow-up is critical to ensure complete resolution of signal changes. Incomplete resolution should prompt the clinician to consider alternative causes for the signal abnormality.
An important factor when considering SRMA is the time taken for these changes to resolve on follow-up. Our findings are based on the timing of the initial and follow-up MRI scans, rather than the initial appearance and the eventual resolution of signal change. This is because serial imaging data, which is required to determine the exact timing of onset and resolution on SRMA, were unavailable. Most patients had only one follow-up MRI scan, several weeks after the baseline scan, while one patient had follow-up MRI two years later. While the available data may not accurately indicate the exact timing, it does provide us with an estimate of when we can expect SRMA to appear and resolve. More accurate quantification would require serial MRIs to be performed on patients, which is an avenue for future investigations.
Hyperintense signal abnormality on a T2-weighed sequence was the most commonly reported manifestation of SRMA. However, there was inconsistency in the type and number of MRI sequences performed and reported. For example, a FLAIR sequence was only performed in seven (26%), while T1-weighted and post-contrast imaging were only performed in eight (30%) and 15 (56%) patients, respectively. Furthermore, DWI was not reported in 14 cases (52%).
When investigating SRMA, an important piece of clinical information to consider is the seizure focus. As mentioned earlier, 71% of generalised seizures exhibited bilateral SRMA and 80% of focal seizures demonstrated unilateral abnormalities. For this review, seizure focus was established based on reported seizure semiology and EEG data. This was challenging due to the inadequate information provided in some cases. Of the 16 focal seizure cases with unilateral SRMA, eight had changes ipsilateral to the seizure focus. In the remaining cases, the laterality of signal abnormality or seizure focus was not specified. Although 25 patients (93%) underwent EEG, minimal information was provided in several cases, and there was inadequate reporting of EEG data in 12 cases (44%). The duration of the EEG recording was not specified in 24 cases (89%), with only three cases (11%) specifying that prolonged EEGs were conducted. In addition, seizure semiology was variably reported; clinical features were inadequate to localise the seizure focus in 12 cases (44%). Unfortunately, this paucity of information limited our ability to localise the seizure focus and draw conclusions regarding the relationship between the seizure focus and SRMA. However, we had sufficient information to distinguish focal-onset seizures from generalised-onset seizures and correlate with MRI abnormalities, providing valuable insights regarding lateralisation. Although we excluded status epilepticus cases from this study, there is evidence in the literature that signal changes can be observed independently from the seizure focus in this group. Nonetheless, it is usually ipsilateral to the seizure focus [3, 19, 22, 23].
Composite patterns were determined through visual analysis of the anatomical location and signal characteristics of SRMA. We noted prominent abnormalities in the hippocampal region - unilateral in six cases, and bilateral in one case. We also observed that SRMA was frequently located in the cortical, subcortical, and leptomeningeal layers, with predominant subcortical, particularly juxtacortical, changes seen in 11 cases (41%) and predominant cortical abnormalities exhibited in four cases (15%). Though temporal lobe seizures are relatively common in clinical practice, we did not find any reference to the involvement of the temporal pole, neocortex, or subcortical regions. This may be due to small sample size. Changes in the splenium were noted in only two cases. The greatest challenge in the deduction of the composite patterns was the lack of MRI images provided by the cases. Fifteen reports (56%) did not provide any images from either the initial MRI scan or the follow-up scan demonstrating resolution.
It is interesting to note that signal changes in the pulvinar nucleus of the thalamus, unilateral or bilateral, are often reported in status epilepticus [24]. In a case series of 32 patients with status epilepticus, MRI signal alterations were detected in mesial temporal structures (17 patients), the pulvinar (16), neocortex (15), and insula (7) whilst exclusive thalamic involvement was seen in three patients [25]. On the contrary, among the patients included in our review, we found only one case with bilateral thalamic T2 hyperintensities in addition to subcortical white matter signal changes [21].
One must be aware of limitations in the clinical interpretation of SRMA. The available data do not indicate that MRI signal changes in this context are reliable for seizure focus localization. This is not surprising given the current understanding of epilepsy as a network disorder, and EEG-functional MRI is likely to be a better tool to investigate seizure-related network dynamics. Some peri-ictal MRI signal abnormalities may reflect neuronal injury in status epilepticus, but pathophysiological interpretations concerning single unprovoked seizures are uncertain. Perhaps the most relevant clinical implication of SRMA is increasing awareness to reduce the risk of misinterpretation of signal abnormalities as the underlying pathological substrate for seizures. It may also help differentiate seizures from mimickers in some cases, but video-EEG monitoring is a more reliable diagnostic tool in clinical practice.
Pathophysiological mechanisms
Localised changes (confined to the region of epileptic discharge) observed in SRMA may be explained by changes in blood flow, excitatory neurotransmitter release, and increased cellular metabolism [17, 26]. The remote changes observed in SRMA are less well understood (e.g. changes in the splenium of the corpus callosum). Seizure activity is associated with a state of increased energy utilisation and the release of excitatory neurotransmitters. Compensatory blood flow increase to the seizure focus occurs to meet this increased metabolic demand. However, a mismatch between energy demand and supply causes cellular stress and increased reliance on anaerobic metabolism [27]. This results in a build-up of lactic acid, carbon dioxide, and free radicals, as well as a reduction in adenosine triphosphate. Downstream sequelae include vasodilatation, loss of neuronal cell membrane integrity due to failure of ion pumps (most notably the sodium-potassium ATPase), intracellular oedema, and activation of various enzymes responsible for cellular apoptosis [4]. Human and animal pathological studies demonstrate localised hypertrophy of dendrites and glial cells, oedema, and sometimes neuronal loss [28].
The hippocampus is particularly vulnerable to cytotoxic damage caused by seizures due to its large number of glutamate receptors [29]. This aligns with the findings of our study, as SRMA was frequently exhibited in the hippocampal region. Additionally, as aforementioned, the results showed a stereotypical pattern of SRMA in subcortical regions of the brain, manifesting with increased T2 signal and rarely low T2 signal.
The increase in T2 and FLAIR signal is thought to be related to vasogenic oedema, which is caused by a net increase in tissue water secondary to increased permeability of the blood-brain barrier [4]. Diffusion restriction (increased DWI signal and decreased ADC signal) reflects cytotoxic oedema [7]. Cytotoxic (or cellular) oedema is associated with an increase in intracellular water and a relative reduction in extracellular fluid secondary to metabolic dysfunction and failure of the cell membrane ion pumps. In seizures, unlike in cerebral ischaemia, the DWI and T2 changes occur roughly synchronously (as opposed to sequentially) suggesting a different mechanism to cerebral ischaemia. Enhancement on post-contrast T1-weighted images likely reflects increased vascular permeability and disruption of blood-brain barrier, causing extravascular leakage of the administered contrast [30]. In this systematic review, T2-hypointense signal abnormality was also reported in one case (4%). Low-intensity subcortical lesions (“dark white matter”) have been described in a number of different diseases, such as malignancy, meningitis, and encephalitis [31]. The exact aetiology is unknown, but it has been postulated to arise from production of ‘a large amount of free radicals’ over a short period of time. The T2-shortening effect of these free radicals may be masked by the competing signal changes caused by vasogenic oedema, potentially explaining why this is not a commonly reported finding in seizures.
Study strengths
We applied strict eligibility criteria to ensure that all selected cases in this study exhibited a complete resolution of SRMA, providing greater certainty in the conclusions. SRMA related to cluster seizures and status epilepticus were excluded to eliminate potential confounders. We also excluded provoked seizures including febrile seizures, as the provoking factor may act as a confounding variable impacting the MRI changes. Animal studies have demonstrated that prolonged febrile seizures result in T2 signal changes on serial MRI without accompanying neuronal injury or death [32]. Furthermore, the publications included both generalised and focal epilepsy cases as well as patients with a first episode of unprovoked seizures. There was also a range of different seizure types included (FAS, FIAS, FBTCS, and GTCS).
Study limitations
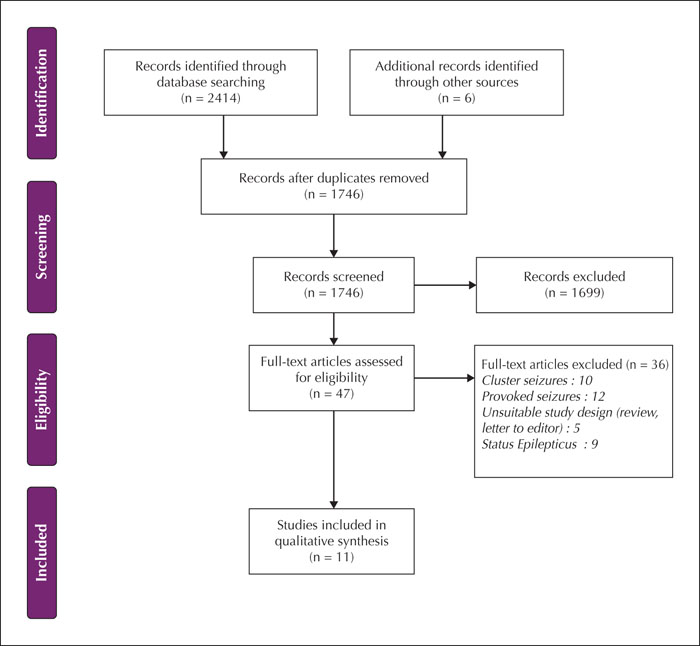
The main limitations of this study stem from the small sample size as well as incomplete and heterogeneous reporting of clinical and radiological data presented in the cases. The final sample included only 11 publications. As highlighted in figure 1, many papers were excluded as those contained cases of provoked seizures, cluster seizures, and status epilepticus. We were unable to define specific time frames because of the way time intervals were reported (many gave a time range). The epilepsy outcomes were rarely reported and the follow-up scan images were not provided in 15 cases (56%). There was also a paucity of information regarding the aetiology, duration of the index seizure, and treatment of the seizures. The duration of the index seizure is particularly relevant as it is likely to influence the cellular and metabolic changes responsible for MRI alterations. Inadequate reporting on seizure focus and semiology limited our ability to draw conclusions about SRMA for different types of seizures.
Additionally, there was marked variability in MRI description and techniques used; not all sequences were performed or reported in all cases, and patients were scanned at different magnetic field strength. Sixteen cases (59%) were reported with 1.5 Tesla (T), one case with 3.0T, another case with 0.5T, and the remaining cases did not specify. In several studies, the standard epilepsy protocol of MRI was not followed. For example, a FLAIR sequence was performed in a quarter of cases, whereas T1-weighted imaging was available in less than a third of patients. Topographic details of imaging abnormalities and the information on the associated structural brain lesions were scant. Due to the lack of ictal EEG data, no inferences could be drawn regarding the lateralizing and localizing value of MRI signal changes.
Time taken for complete resolution of MRI abnormalities could not be ascertained with certainty due to the lack of serial scanning. Another limitation of this review is the small number of cases that satisfied the eligibility criteria. The 11 papers that were deemed suitable yielded a total of 66 cases, but only 27 cases satisfied the eligibility criteria as we applied those criteria to each individual case to screen for eligibility. The use of these strict eligibility criteria was, however, important for reducing the effect of potential confounders and increasing reliability of the findings. There were no comparator groups among any of the published studies. Therefore, we are unable to draw any conclusions on how to differentiate SRMA from other reversible MRI lesions, such as leptomeningeal vasculitis.
Gaps in knowledge
There are still many unanswered questions in the realm of SRMA. The cause and pathophysiology of SRMA are incompletely understood. Additionally, the association between signal changes and the seizure focus is also not clear, although we were able to make some preliminary deductions based on the limited information available. Furthermore, data on the timing between the seizure and initial appearance and eventual resolution of SRMA remain to be precisely elucidated.
Future directions
A more extensive analysis of the clinical data of SRMA would be possible through prospective studies with larger sample sizes involving multiple seizure types. It would also be beneficial to look at the characteristics of SRMA in different seizure presentations, including status epilepticus and cluster seizures, and study how the underlying aetiology might impact on these findings. It would also be worthwhile evaluating whether SRMA has any value for prognostication and assessment of treatment response through longitudinal studies. Systematic, serial scanning of patients at clearly defined time-points would allow us to more accurately define time frames for both the appearance and resolution of SRMA.
Conclusions
Clinicians should be aware that SRMA describes a heterogeneous collection of radiologic findings with identifiable stereotypic patterns. These changes may be present within hours of seizure onset and appear to resolve in a relatively short time frame in some cases. SRMA are commonly observed in the subcortical layers and the hippocampi, but may also be seen in the cortex and the splenium. Bilateral SRMA may be associated with generalised seizures while unilateral SRMA may be associated with focal seizures and may occur ipsilateral to the seizure focus. Systematic and standardised reporting of clinical and radiologic data is necessary to allow a more accurate definition of the characteristics of SRMA.
Supplementary material
Summary slides accompanying the manuscript and supplementary materials are available at www.epilepticdisorders.com.
Disclosures
We know of no conflicts of interest associated with this manuscript, and there has been no significant financial support for this work that could have influenced its outcome.