Epileptic Disorders
MENUPost-encephalitic epilepsy in childhood: results from a prospective cohort study Volume 23, issue 1, February 2021
Key points
Encephalitis is a potentially devastating condition with an annual incidence ranging from 8 to 16/100,000 children worldwide [1, 2]. The long-term outcome after encephalitis in childhood varies from death or severe sequelae to full recovery [3-5]. Prognosis is complicated by a weak relationship between the clinical picture in the acute phase and the prevalence of remaining sequelae. Some correlation is seen with a worse outcome in agents that cause necrosis or vasculitis, such as herpes virus, but the individual variation for a given aetiology is large. Seizures are common during acute encephalitis and may be even more so in children [4, 6]. Progression of seizures to status epilepticus, requiring pharmacological treatment, is also common and seen in up to 40% of adult patients [6-8]. Adult patients with viral encephalitis who present with seizures are at substantially higher risk (up to 22-fold) of developing recurrent seizures post-infection [9, 10]. A retrospective study on adult patients showed that the main risk factors of developing post-encephalitic epilepsy (PEE) were acute seizures and neuroradiological abnormalities [11]. Following the acute phase of encephalitis, antiepileptic drugs (AEDs) are often maintained for a long time to prevent recurrent seizures. However, many patients will never have unprovoked seizures after the acute phase and long-term AED treatment may not be warranted [7, 12, 13]. Although long-term use of AEDs may prevent possible unprovoked seizures in the future, it may also have a negative impact on brain development and reduce quality of life [14-16]. The risk of seizure recurrence and the development of post-encephalitic epilepsy therefore needs to be weighed against the potential risks and negative effects of AED treatment. However, the prevalence of post-encephalitic epilepsy in children is difficult to ascertain as previous studies have been limited by lack of clinical data, important risk factors for developing epilepsy or the use of highly selected cohorts from tertiary centres.
We studied acute clinical risk factors for developing PEE. Using several identified risk factors, we created a simple algorithm that could be used to identify patients with increased risk of PEE.
Methods
Study design
Children aged 28 days to 17 years, who were admitted to a primary and tertiary care hospital in northern Stockholm, between May 2011 and May 2016, due to acute encephalitis, were included in the cohort (n=89). The diagnosis of encephalitis was based on the following criteria:
- –Signs of cerebral dysfunction either as:
- •encephalopathy defined as altered consciousness, personality or behavioural changes lasting for more than 24 hours, or
- •abnormal electroencephalography (EEG) findings compatible with encephalitis, plus at least one of the following: abnormal results of neuroimaging compatible with encephalitis, positive focal neurological findings or seizures.
- –Signs of inflammation, defined either as cerebrospinal fluid (CSF) pleocytosis (≥6 white blood cells/μL), fever (>38̊C), elevated infectious parameters (C-reactive protein and/or white blood cells [WBC)) or presence of neuronal antibodies.
Children with another verified cause of symptoms, such as bacterial meningitis or other known underlying disease that per se could explain the symptoms, were excluded. Pure ataxia was not considered a sufficient neurological sign for inclusion. Demographic characteristics, clinical signs and symptoms were recorded for all children.
Laboratory examinations
Blood and CSF were prospectively sampled according to the study protocol as well as when clinically motivated. All children underwent routine laboratory tests at the time of admission including routine clinical infection workup and CSF analysis. Antibody titres in blood were analysed for Borrelia burgdorferi and tick borne encephalitis (TBE) virus in all patients, and depending on clinical symptoms, some cases were tested for enterovirus, adenovirus, Mycoplasma pneumoniae, herpes simplex virus type 1 and 2 (HSV1 and HSV2) and varicella zoster virus (VZV). Lumbar puncture was performed during the acute phase with routine analysis of the CSF, including WBC, levels of albumin, glucose, and lactate and microbiological analyses. Microbiological analysis of the CSF included bacterial culture and virological tests for HSV1, HSV2, VZV and enterovirus with polymerase chain reaction (PCR). Depending on the season and clinical picture, the CSF was also tested for other aetiologies, such as Borrelia burgdorferi, human herpes virus 6, parechovirus, cytomegalovirus and Epstein Barr virus in some cases. Tests for antibodies directed at neuronal antigens, such as N-methyl-D-aspartate receptor (NMDAR) antibodies, were not routinely performed. However, in children in whom the initial aetiological screening was negative, and no clinical improvement was seen or following relapse, tests for neuronal antibodies were considered. Nasopharyngeal aspirate was tested for respiratory viruses when respiratory symptoms were present. Faeces were analysed with PCR for enterovirus, and if gastrointestinal symptoms were present, most children were also tested for norovirus, sapovirus and rotavirus. The detection of a viral agent in the CSF or intrathecal production of antibodies and the presence of antibodies against TBE virus in serum was defined as confirmed aetiologies, whereas other agents found in the blood, faeces or nasopharynx were labelled as probable aetiologies.
EEG recording
All children included in the study underwent a 30-minute EEG examination within the first days of admission. This was then repeated as clinically indicated during the admission. The following EEG abnormalities were noted: background and focal and epileptic (interictal and ictal) abnormalities.
Neuroradiology
In children who underwent neuroimaging, computerised tomography (CT) or magnetic resonance imaging (MRI), new-onset structural lesions were noted.
Post-encephalitic epilepsy
PEE was assessed at 24 months after admission to hospital. Predisposing factors for epilepsy as well as factors from initial presentation and hospital course were assessed. Medical records were reviewed including all prior and subsequent clinic visits, medications administrated, laboratory results, neuroimaging studies, and EEG results. At the 24-month follow-up visit, all unprovoked seizures during the 24 months following hospital discharge were noted. The diagnosis of epilepsy was given to patients with at least one unprovoked seizure after resolution of the acute insult, which is in accordance with international standards [17, 18]. We also studied the estimated seizure burden, type and number of AEDs given during the acute phase of illness, at six months and 24 months post first hospital admission.
Statistical analysis
Mean and standard deviation of continuous variables and counts and percentages of categorical data are reported. Fisher's exact test was used to examine the association between categorical variables and Mann-Whitney U-test was used to compare groups of continuous variables. A p value less than 0.05 was considered statistically significant. Tests used to predict PEE were assessed together with estimated probability of PEE with 95% confidence intervals (Wald test). All statistical analyses were performed in MATLAB®(The MathWorks, Inc., Natick, Massachusetts, United States).
Ethical approval
This study was approved by the local ethics committee (Dnr 2010/1206-31/1). All guardians of patients and patients older than 15 years of age provided written informed consent.
Results
Post-encephalitic epilepsy
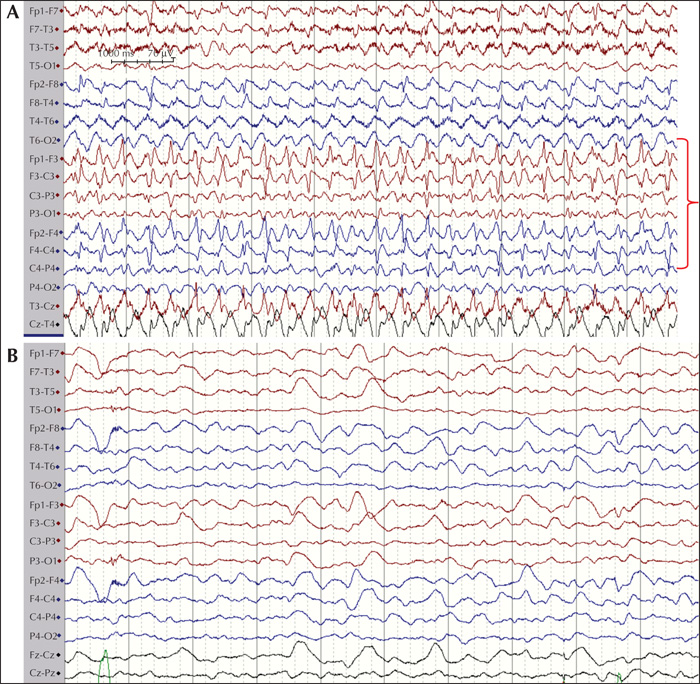
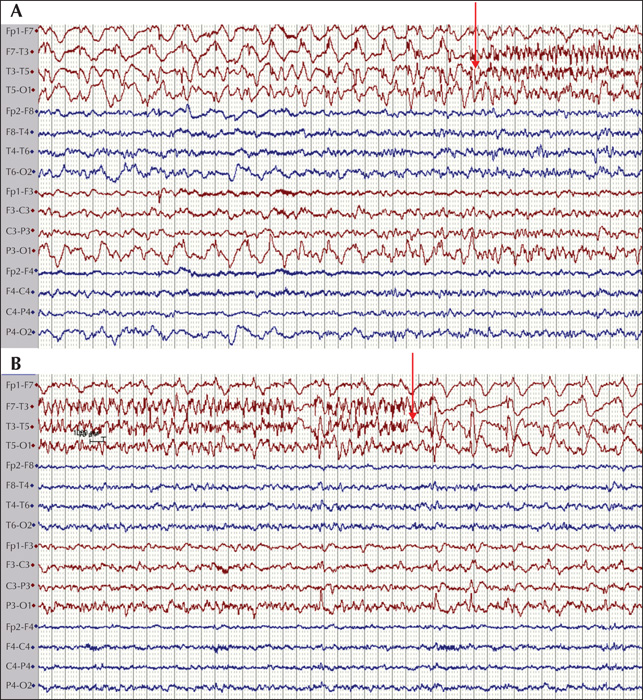
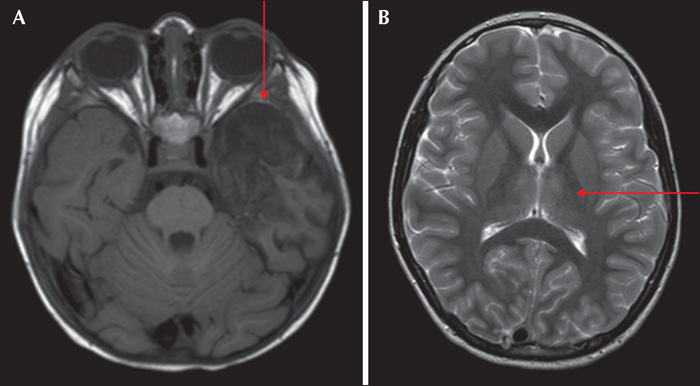
The final cohort consisted of 89 children (46 females and 43 males) who fulfilled encephalitis criteria; 87 of these had EEG recordings available for classification. The median age at presentation was 72.4 months (range: 1-207 months). When evaluated at six months, eight patients had at least one seizure post resolution of the acute insult. Of these, four patients (50%) were on monotherapy (oxacarbazepine, levetiracetam and lamotrigine) and four (50%) were on polytherapy, including three patients (37.5%) who were medically refractory. The following AEDs were used in polytherapy in different combinations at six months: clobazam, lacosamide, levetiracetam, oxcarbazepine, phenytoin, and valproate. At 24 months, no new patients had any seizures and the same eight patients identified at six months were given the diagnosis PEE. At 24 months, three (37.5%) were on monotherapy and five (62.5%) on polytherapy. Of the patients on polytherapy, three were refractory. The following AEDs were used in polytherapy in different combinations: clobazam, zonizamide, levetiracetam, oxcarbazepine, topiramate, phenytoin, and valproate. Three patients with PEE were well controlled on AED treatment but had seizure breakthrough on drug reduction. Two patients were well controlled on AEDs but had breakthrough seizures during infection, which caused only occasional seizures. The three remaining patients had at least one seizure per week. Two patients had primary (Hashimoto's encephalitis [HE]) or secondary autoimmune (HSV-1 to NMDA-R-Ab) encephalitis with epilepsy, see figures 1 and 2 for EEG during the acute phase of the disease. The patient with HE was treated with IV immunoglobulin, followed by IV methylprednisolone and then tapered onto a sustenance dose of oral prednisolone. This patient was sensitive to reduction in the prednisolone dose with breakthrough seizures (HE) and was then treated aggressively with IV immunoglobulin repeatedly. The patient with NMDA-R Ab encephalitis was treated with high-dose prednisolone with tapering to a sustenance dose. The patient with HE did not show any oligoclonal bands in the CSF, however, these were seen in the patient with NMDA-R-Ab encephalitis. The eight patients who developed PEE had, during the acute phase of the disease, status epilepticus (50%), multiple seizures (37.5%) or a single acute seizure (12.5%). Most acute seizures were focal with lateralising features (asymmetric motor; 62.5%) (table 1). Asymmetric MRI findings were found in three patients with focal acute seizures in which focal abnormalities were seen in the insula or temporal lobe (see figure 3 for two cases with MRI abnormalities).
Risk factors for developing post-encephalitic epilepsy
Aetiology
Different types of etiological agents were identified (n=18) and four of these were each present in more than five patients: enterovirus, TBE virus, VZV and rotavirus. There was no association between these four etiological agents and development of PEE. Similarly, patients with an unknown etiological agent did not show any association with development of PEE at 24 months (table 2).
Acute seizures
Acute seizures were seen in all patients who developed PEE and in 28 who did not develop PEE. There was a significant association between acute seizures and the development of PEE (OR = 15, p<0.001 [Fishers Exact test]). Presence of multiple acute seizures or epileptic status epilepticus was seen in seven patients who developed PEE and 13 who did not (OR=37, p<0.001). The presence of status epilepticus was also statistically associated with the development of PEE (OR=11, p<0.01) (table 2).
Neuroradiology
At 24 months, five patients with structural abnormalities of the brain had developed PEE while nine did not show any significant association (OR=6, p<0.05) (table 2).
EEG
The EEGs during the acute illness were analysed for background as well as focal and epileptiform (inter-ictal and ictal) abnormalities. In 68 patients, changes in background activity were seen, and of these, eight developed PEE whereas 60 did not. Focal abnormalities were present in 21 patients, of whom four developed PEE and 17 did not. These parameters were not statistically associated with risk of developing PEE. Epileptic abnormalities (interictal spikes/sharp-waves and electrographic seizure activity) were seen in six patients who developed PEE and seven who did not, and were thus significantly associated with PEE (OR = 31 and p<0.001 [Fishers exact test]) (table 2).
Cerebrospinal fluid analysis
Patients were examined for the following parameters based on cerebrospinal fluid: albumin concentration, monocytes and polycytes. None of these parameters were significantly different between the group that developed PEE and the group that did not develop PEE (Mann-Whitney U test) (table 2).
Hospital admission and intensive care
Patients who developed PEE had a longer median hospital admission (23.5 vs. 8.6 days) and longer duration in intensive care (3.2 vs. 2.2 days). These medians were significantly different for hospital admission (p<0.01, Mann-Whitney U test), but not for stay in intensive care (table 2).
Algorithm for cohorting patients with increased risk of PEE
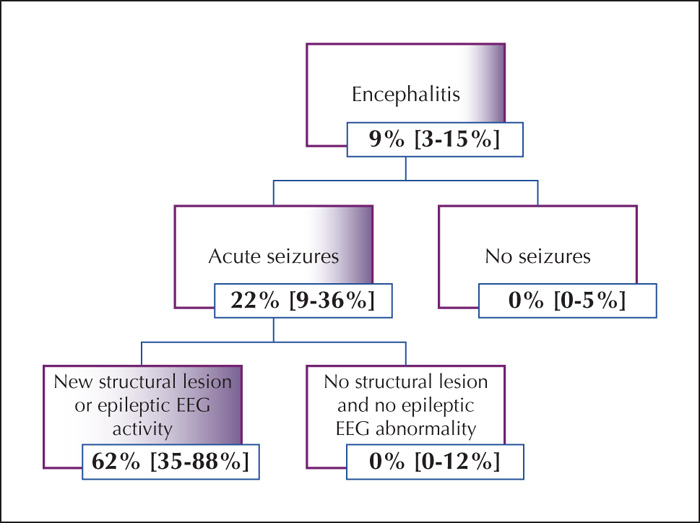
We developed an algorithm to cohort patients into groups of increased risk of developing PEE (figure 4). Based on the algorithm, acute seizures were seen in all patients who later developed PEE. Within this group, the risk of developing PEE was estimated at 22% (95% confidence interval 9-36%). Further, inclusion of new structural lesions or epileptic activity on EEG resulted in a subgroup with a risk of epilepsy estimated at 62% (95% confidence interval 35-88%) (figure 1).
Discussion
Development of PEE is of serious concern and increases the resultant long-term morbidity. Treatment with AEDs can often control seizures but may sometimes lead to several adverse reactions including impaired cognitive function and fatigue. In this study, the risk of developing PEE was investigated and several parameters, defined during the acute insult, were used to stratify the risk. These were related to the presence of clinical, electroclinical or neuroradiological attributes of seizures and increased the odds ratio by approximately 5-35, i.e. presentation with acute seizures, epileptic activity on EEG and structural lesions on brain MRI or CT. Similar findings have been described previously in PEE and also for specific types of autoimmune encephalitis [19-21]. The presence of acute seizures showed high sensitivity for developing PEE while the presence of epileptic activity and presence of new structural lesions instead showed lower sensitivity but higher specificity for developing PEE. Using these acute parameters, we created an algorithm for cohorting patients into groups with a high risk of developing PEE.
In this prospective study, approximately 9% of patients developed PEE which is substantially lower than what has been seen in previous studies [5, 6, 11, 21]. This risk has been shown to be related to the aetiology in which certain viral agents have shown high probability of developing PEE e.g. 75% for herpes encephalitis [6, 20, 22]. PEE has been shown to vary considerably for patients with autoimmune encephalitidis for which the risk depends on the specific antibody present. N-methyl-D-aspartate receptor antibodies have been shown to be associated with a high rate of acute seizures but low level of PEE, in contrast to anti-MOG encephalitis in which approximately 16% of patients develop PEE [7, 21, 23, 24]. For the diagnosis of epilepsy, it is important that seizures occur spontaneously and that they are not part of the acute inflammatory insult. However, as there is no time limit following an acute episode of encephalitis, defining when a subsequent seizure should be considered unprovoked as opposed to part of an unresolved inflammatory response, the definition may vary. Several studies have assessed PEE at six months while other studies have assessed it at 24 months. In the present study, we have estimated the presence of PEE at 24 months post encephalitis. The present cohort, however, was different in several aspects to earlier studies as the hospital admission setting allowed for an unbiased estimate within the general paediatric population without bias towards complicated cases, as often seen in tertiary referral centres. The spectrum of viral agents was different with only a minor part being caused by highly epileptogenic agents such as HSV1 and 2. In comparison to the risk of developing PEE due to autoimmune encephalitis, we found a lower level, 9% (but with confidence interval between 3-15%) vs 16%, again indicating different PEE prevalences with different mixtures of aetiology. On examining only patients with autoimmune encephalitis in this study, we estimated the risk of developing PEE to approximately 30% which is inline with previous studies, although the estimation of the risk may not be accurate due to the limited number of patients with autoimmune encephalitides [21]. Two patients with autoimmune encephalitides and PEE (NMDA-R-Ab and HE) were treated with a combination of AED and immune-modulators. Both of these patients showed sensitivity for reduction of immunomodulating therapy which resulted in breakthrough seizures. This was more evident for the patient with HE who developed status epilepticus on several occasions due to reduction of prednisolone and was treated with IV immunoglobulin, as has been described previously in HE [25].
This and several previous studies would indicate that PEE develops mainly within the subgroup of patients who have seizures during the acute phase of the disease. Moreover, presence of electrographic epileptic activity or new-onset structural lesions increases the risk further. Further dissection of the inflammatory processes might reveal the process or processes that lead to PEE with the possibility of future intervention.
This study has strengths and limitations. The prospective design with standardized time points for data acquisition and sampling increases the likelihood of finding true associations and removes the risk for recall bias and interpretation of retrospective charts. Another strength is that the cohort comes from a centre serving a primary catchment area, leading to a mixture of aetiologies likely to be representative of the general population. This may be of importance as studies of cohorts from centres that mainly serve as tertiary referral centres may accumulate more complex cases and therefore overestimate the associated risks. Limitations include the heterogeneous cohort spanning over both age and aetiology. Although the total number of children in the study was fairly large, the number of patients of a certain age with a certain aetiology was low.
Factors affecting the risk of developing epilepsy following encephalitis in childhood are important to support correct prognostication and consequent use of prophylactic treatment. As such factors have not been determined, there is no standardized approach for the management of these patients concerning who to treat, what AEDs to use or the duration of treatment. This study provides a simple tool (algorithm) for determining the risk of PEE in children following the acute event which may be used for clinical decision-making.
Acknowledgements and disclosures
Funding was obtained from Stockholm City Council, The Jerring Foundation, Stiftelsen Samariten, Sällskapet Barnavård, Linnea and Josef Carlssons Foundation, the IKEA Foundation and Karolinska Institutet. None of the authors have any conflict of interest to declare.