Epileptic Disorders
MENUPathophysiology of encephalopathy related to continuous spike and waves during sleep: the contribution of neuroimaging Volume 21, supplement 1, June 2019
Figures
Epileptic encephalopathy with continuous spikes and waves during slow sleep (ECSWS) is an age-related disorder characterized by acquired variable neuropsychological impairment, epilepsy with heterogeneous seizure types, and the presence of the interictal electroencephalographic (EEG) findings of intense sub-continuous paroxysmal activity of spike-wave complexes that usually occupy more than 85% of non-REM sleep (Tassinari et al., 2005). ECSWS can be attributed to different etiologies (symptomatic cases with various structural brain lesions, and MRI-negative cases with probable genetic background, for example, in the form of Landau-Kleffner syndrome LKS or atypical benign partial epilepsy of childhood) and is associated in the majority of cases with manifold acquired psychomotor and cognitive deficits and even regressions (auditory agnosia, acquired aphasia, dysfunctions of the frontal lobe and short-term memory deficits, pseudo-bulbar palsy, global mental deterioration, impaired spatial orientation, apraxia and hemineglect, psychotic states and autistic features, attention deficit and hyperactivity and aggressiveness). Despite its significance, very little is known about pathophysiological mechanisms of ECSWS. Here, we summarize studies on functional neuroimaging which shed light on the pathophysiology of this defacing condition.
Source reconstruction
The first neuroimaging studies focused on the source reconstruction of epileptic spikes in patients with ECSWS and LKS based on EEG and MEG data. There is a general agreement that the perisylvian cortex is an important part of the network in patients with ECSWS (Morrell et al., 1995; Paetau et al., 1999; Sobel et al., 2000; Siniatchkin et al., 2010; Shirashi et al., 2014; De Tiege et al., 2013). In most cases with ECSWS, the perisylvian sources were found bilaterally, whereas in some patients with LKS, most sources were left-sided. The importance of the perisylvian cortex to trigger ECSWS is supported by clinical observations (frequent occurrence of ECSWS in patients with perisylvian polymicrogyria and association of structural abnormalities in the perisylvian region with LKS and ECSWS) and some treatment studies (encouraging results from multiple subpial transections in the perisylvian region in the treatment of ECSWS) (Morrell et al., 1995). It seems likely, however, that the perisylvian cortex is not always the generator of epileptic activity, even in patients with LKS (Paetau et al., 1999). In those cases where the generator is located in other cortical areas, the epileptic activity may propagate bilaterally to the perisylvian cortex (Morrell et al., 1995; Siniatchkin et al., 2010; De Tiege et al., 2013).
PET studies
The first PET studies were performed during sleep and wakefulness by Maquet et al. (1995) who investigated 6 patients with ECSWS of different etiology using /18F/fluorodeoxyglucose (FDG). They showed unilateral, focal or regional cortical increases in glucose metabolism during both sleep and wakefulness. The most cortical areas associated with CSWS included perisylvian, superior temporal and inferior parietal areas. The metabolism in the cortical mantle was higher than in the subcortical regions. Interestingly, after successful treatment of ECSWS and recovery of symptoms, the described metabolic changes disappeared. The study of Maquet et al. provided important insight into the pathophysiology of ECSWS and enabled the following conclusions which were supported later by other studies:
- –It seems likely that the associative cortex, especially the perisylvian and superior temporal regions, are involved in the generation and propagation of epileptic activity in CSWS. Although the study provides strong evidence for an individual focal cerebral dysfunction in CSWS, systemic abnormalities such as pathological activity in the thalamo-cortical network may play a significant role in this pathological condition.
- –Similar metabolic changes in the brain may be found during sleep and in wakefulness. This fact may explain why patients with dramatic activation of epileptic activity during sleep develop cognitive deficits during the day, when the epileptic activity is less pronounced or may even disappear.
- –The described pathological changes represent a functional fingerprint of ECSWS, independent of etiology.
These results were confirmed by the Brussels group in a number of publications. In these studies, data were analysed using a voxel-based statistical methodology that enabled group analyses and connectivity studies in addition to individual analyses. De Tiege et al. (2004) investigated 18 patients with ECSWS during wakefulness using FDG-PET and analyzed data at the individual level and at the group level using a group control of healthy young adults. At the individual level, they found areas of hypermetabolism in the cerebral cortex, clearly well associated with the focus of interictal epileptic activity. At the group level, hypermetabolic areas involved the postcentral gyrus and parietotemporal junction very close to the perisylvian region. In addition, areas of hypometabolism were found in most cases in the prefrontal and frontal cortex. Also at the group level, altered functional connectivity was found between hypermetabolic (parietal) and hypometabolic (frontal) brain regions and was interpreted as reflecting remote inhibition of frontal areas through epileptic activity as a possible explanation for cognitive deficits in affected individuals. These data were recently confirmed using a pediatric population as pseudo-controls, showing at the group level hypometabolism in regions that belong to the default network (prefrontal and posterior cingulate cortices, parahippocampal gyrus and precuneus) (Ligot et al., 2014). Later, De Tiege et al. (2008) investigated nine patients with ECSWS during the acute phase of the disease and recovery. Again, the authors described areas of focal hypermetabolism in the centro-parietal regions and right fusiform gyrus and widespread hypometabolism in the prefrontal and orbitofrontal cortices, temporal lobes, left parietal cortex, precuneus and cerebellum. Both hyper- and hypometabolism markedly regressed at recovery. These studies provided the evidence that the metabolic effects of CSWS activity and its associated neuropsychological impairements are not restricted to the epileptic foci but spread via the inhibition of remote neurons within connected brain areas. Finally, De Tiege et al. (2013) combined FDG-PET and source reconstruction in six patients with ECSWS. The areas of hypermetabolism were associated with the onset or propagation site of epileptiform discharges as revealed by the source analysis. Areas of hypometabolism were not related to epileptic activity.
Recently, Agarwal et al. (2016) studied glucose metabolism in 23 children with CSWS using FDG-PET with a specific focus on metabolic changes in the thalamus. Thalamic glucose metabolism was abnormal in 78,3% of patients. However, the abnormalities were very inhomogeneous. Some children were characterized by unilateral, some by bilateral, metabolic abnormalities, some of them demonstrated abnormal increases and some children showed decreases in metabolism. There was no clear association between thalamic metabolic abnormalities and the stage of evolution of ECSWS (prodromal, acute, or residual). Although determining the relevance of thalamic metabolic abnormalities in the pathogenesis of CSWS for any particular patient is challenging, the study emphasizes the role of thalamic pathology and significance of thalamo-cortical network in mechanisms of ECSWS (Agarwal et al., 2016).
In summary, PET studies have provided evidence for typical functional brain abnormalities in patients with ECSWS which (1) are characterized by the association with cortical focal hypermetabolism and hypometabolism in distinct connected cortical areas (the significance of thalamo-cortical network was underlined in several studies), (2) remain to be stable during wakefulness and sleep, and (3) are largely reversible at recovery of ECSWS, indicating that these abnormalities are attributed to epileptic activity. It is unclear whether these abnormalities are related to the increase of epileptic activity during sleep. One possible way to investigate this question is to study correlations between epileptiform discharges and neurometabolic / haemodynamic changes in the brain using simultaneous recordings of EEG and functional MRI (EEG-fMRI).
EEG-fMRI studies
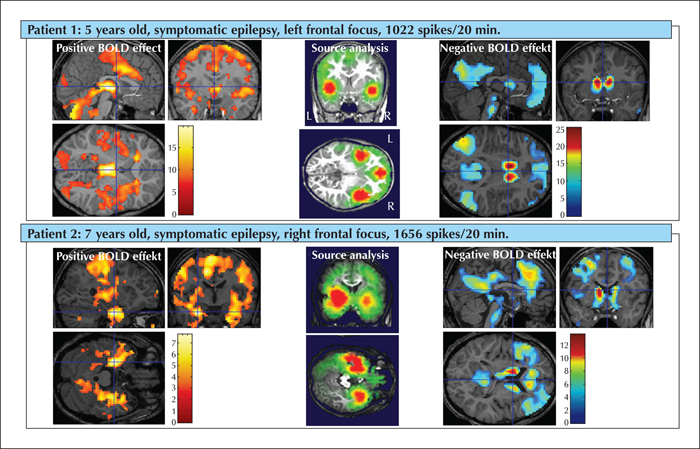
The first EEG-fMRI study on CSWS was published by De Tiege et al. (2007). The authors investigated a nine-year-old girl suffering from partial seizures and who developed CSWS and neuropsychological deficits. Epileptiform activity was associated with focal activation in the right superior frontal, postcentral, and superior temporal cortex as well as deactivation in the lateral and medial frontoparietal cortices, posterior cingulate gyrus and cerebellum. In concordance with this study, Siniatchkin et al. (2010) investigated 12 children with ECSWS of different etiologies using simultaneous recordings of EEG and fMRI. The study revealed a typical network of brain activation in patients: the positive BOLD signal changes involved the bilateral perisylvian region (which was clear based on PET and MEG/EEG studies) and cingulate gyrus as well as bilateral frontal and parietal cortex and thalamus. Electrical source analysis demonstrated a similar involvement of the perisylvian brain regions, independent of etiology and area of spike generation. Moreover, the source reconstruction provided evidence that the typical pattern of brain activation is more likely to be related to a specific pattern of propagation of epileptic activity during ECSWS (figure 1). Negative BOLD signal changes were identified in the precuneus, lateral parietal cortex and medial frontal cortex. These structures are usually involved in a pattern of deactivation that occurs during the initiation of task-related activity and represent a default mode network (DMN) which is active in the resting brain with a high degree of functional connectivity (Raichle et al., 2001). It has been suggested that the DMN constitutes a necessary favourable neurometabolic environment for cognitive functions, represents a physiological baseline for processes of attention and working memory, and supports dynamic integration of cognitive and emotional processing (Raichle and Mintun, 2006). Abnormal activity in the DMN and disturbed connectivity between the structures involved may influence task performance and contribute to pathogenesis of neuropsychiatric disorders such as attention-deficit hyperactivity, Alzheimer's disease, autism, schizophrenia, and depression (Broyd et al., 2009). It has been suggested that disruption of the resting state activity by pathological processes (e.g. those that give rise to spikes) may be related to alterations in cognitive function and this may be a possible mechanism which may underlie cognitive deficits in epilepsy (Gotman et al., 2005). Deactivation in the DMN has been described in patients with primary and secondary generalized paroxysms and absence seizures as well as in patients with temporal lobe epilepsy (for a review, see Moeller et al. [2013]). The authors hypothesized that one of the possible mechanisms to explain how epileptic activity in patients with ECSWS causes cognitive deficits is the mechanism of interruption of the activity and connectivity in the DFM. Note that areas of hypometabolism revealed by PET studies resemble those of deactivation in EEG-fMRI studies representing remote inhibition of the default mode network (Ligot et al., 2014). This is an elegant hypothesis to explain cognitive deficits by inhibition/deactivation in the DMN. However, this hypothesis should be supported by neuropsychological and neuroimaging data, which has not yet been performed.
Functional and effective connectivity
There is another explanation for the interaction between the DMN and epileptic activity. Based on analysis of effective connectivity in patients with absence epilepsy, Vaudano et al. (2009) provided evidence that activity in the precuneus, as part of the DMN, gates spike-and-wave discharges in the thalamo-cortical network. It seems likely that haemodynamic changes in the precuneus, as an index of awareness and fluctuations of vigilance, trigger epileptic paroxysms (Vaudano et al., 2009). The question whether the epileptic network causes changes in the DMN or vice versa was explored in the recent study of Japaridze et al. (2016). Sleep EEGs before and after treatment were investigated in fifteen patients with ECSWS (including three patients with Landau-Kleffner syndrome). In order to study functional and effective connectivity within the network generating the delta activity in background sleep EEG, the methods of dynamic imaging of coherent sources (DICS, a method of source analysis in frequency domain) and renormalized partial directed coherence (RPDC, a measure of causality between sources of activity) were applied. Independent of etiology and severity of epilepsy, the background EEG pattern in patients with ECSWS before treatment was associated with the complex network of coherent sources in the medial prefrontal cortex, somatosensory association cortex/ posterior cingulate cortex, medial prefrontal cortex, middle temporal gyrus/ parahippocampal gyrus/ insular cortex, thalamus, and cerebellum (based on both individual as well as group level). The analysis of information flow within this network revealed the medial parietal cortex and precuneus as well as thalamus as central hubs, driving the information flow to other areas, especially to the temporal cortex where epileptic activity originates. The described CSWS-specific pattern of functional and effective connectivity was no longer observed in patients with normalized sleep EEG after a successful treatment. The study demonstrates the leading role of the precuneus and thalamus in the hierarchical organization of the network underlying the background EEG in CSWS and points towards the significance of fluctuations of vigilance in the generation of CSWS. This hierarchical network organization seems to be specific for CSWS as it resolves after successful treatment (Japaridze et al., 2016).
Conclusions
Functional neuroimaging represents a powerful methodology to investigate pathogenic mechanisms of epileptic encephalopathies including ECSWS. Recent studies demonstrated that in patients with ECSWS, different etiological factors correlate with the same pattern of network activation in areas including the perisylvian region, temporal, parietal and cingulate cortex. It seems likely that this CSWS-specific network represents a pattern of propagation of epileptic activity. Moreover, besides the network related to epileptiform discharges, there are changes in brain activity remote from the epileptic focus. These remote areas of reduction of brain activity include the precuneus, parietal and medial frontal cortex - brain areas of the default mode network. It could be that the epileptic activity interferes with the DMN and, in such a way, influences cognitive function. According to this hypothesis, the remote inhibition may possibly explain cognitive deficits in patients with CSWS. However, it could also be that the fluctuation of activity in the DMN facilitate generation of epileptiform discharges. This hypothesis would explain an increase of epileptic activity during sleep in patients with CSWS. The described abnormalities are functional in nature and disappear after recovery of symptoms following successful treatment. Whatever the explanation, confirmatory studies are needed in order to explore the interaction between epileptic and other functional networks in patients with ECSWS.
Disclosures
None of the authors have any conflict of interest to declare.