Epileptic Disorders
MENULate-onset epileptic spasms may be cured by focal cortical resective surgery Volume 14, issue 3, September 2012
epd.2012.0523
Auteur(s) : Brigitte Ricard-Mousnier1,,2 BrRicard-Mousnier@chu-angers.fr, Georg Dorfmuller3,4, Martine Fohlen4, Christine Jeanguillaume5, Silvie Nguyen2, Olivier Delalande4, Christine Bulteau3,4
1 Centre Hospitalier Universitaire, Neurology Department, Angers
2 Centre Hospitalier Universitaire, Pediatric Department, Angers
3 Inserm U663, University Paris Descartes, Paris
4 Fondation Ophtalmologique A de Rothschild, Pediatric Neurosurgery Department, Paris
5 Centre Hospitalier Universitaire, Service de Médecine Nucléaire, Angers, France
Correspondence: Brigitte Ricard-Mousnier Service de Neurologie, CHU 4, rue Larrey, 49033 Angers cedex 01, France
Late-onset epileptic spasms (ES) form a heterogeneous group (Gobbi et al., 2007). They may be associated with various types of seizures, such as partial seizures in partial cryptogenic or symptomatic epilepsies, and some epileptic encephalopathies. We report the case of a child with isolated late-onset ES who experienced a substantial improvement following focal frontal cortectomy.
Case report
A girl, born in 1991, had no significant perinatal history and early psychomotor development was normal. Clusters of axial and limb tonic contractions with head nodding were observed by the family at 2 years of age. The clusters occurred only during sleep and lasted 15 to 20 minutes. Neurological examination and awake EEG were normal. Valproate was administered. The girl continued to have clusters, two or three times a week. In the absence of any identified epileptic origin, treatment with valproate was stopped and the frequency of the clusters was unchanged.
Sleep EEG at the age of 5 years revealed clusters, leading to the diagnosis of epileptic seizures. Interictal EEG showed left frontal spikes during slow sleep. Ictal EEG revealed generalised biphasic slow waves with superimposed fast rhythms lasting 0.5 to 1 second during axial hypertony, repeated for 10 minutes, and occurring throughout the cluster. Several antiepileptic drugs (carbamazepine, vigabatrin, clonazepam, lamotrigine, topiramate, levetiracetam, and oxcarbamazepine, successively) were tried and ineffective.
Educational difficulties were noted at the age of 7 years, mostly in mathematics. Neuropsychological assessment performed at the age of 10 years showed mild intellectual disability (WISC-III: TIQ: 62) and the girl was oriented towards an adapted school.
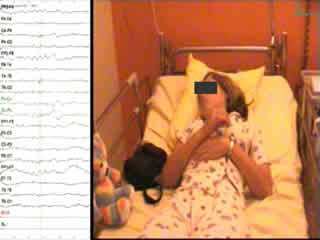
A second video-EEG was performed at 14 years of age and a cluster of seizures was recorded during sleep; the patient woke and presented with repeated episodes of sudden head flexion with abduction of the upper limbs and flexion of the lower limbs. The first events were very brief and symmetric. The following motor sequences became longer (lasting 1 to 1.5 seconds) with a tonic component and were asymmetric, the movement of abduction being larger on the right limbs. The patient also had a hiccup (see video sequence). The last events of the episode were brief, similar to the start, but remained asymmetric. The frequency and intensity of the events in the cluster increased and then slowly decreased .The cluster lasted 16 minutes. During the cluster, consciousness was preserved and language was normal. During the first events, EEG showed a diffuse high-voltage sharp-and-slow-wave complex, then, as the events became asymmetric and more tonic, a period of voltage attenuation followed the slow-wave pattern which was generally diffuse, symmetric or asymmetric, longer and more evident over left central and temporal regions. The localisation of the slow-wave pattern was diffuse with a maximum peak over the vertex region. Between these events, there was a diffuse theta activity and left frontotemporal delta waves. Interictal activity was normal during the awake state. During slow sleep, there were bursts of generalised asymmetric slow spikes and waves predominantly on the left frontotemporal area (figure 1), as well as generalised, symmetric, synchronous slow spikes and waves, in some cases interrupted by slow polyspikes and waves.
FDG-TEP-MRI co-recording demonstrated a localised hypometabolic area over the medial part of the left superior frontal gyrus and a corresponding signal abnormality on MRI (figure 2). Because of this concordant result, a stereoelectroencephalography (SEEG) investigation was performed using intracranial multiple contact electrodes. Seven left frontal electrodes and three left temporal electrodes were placed as shown in figure 3. Interictal SEEG during the awake state showed multifocal spikes, polyspikes, and spike-waves recorded asynchronously from all electrodes; these discharges became generalised and synchronous during slow sleep. Three infraclinical rhythmic sharp wave discharges were recorded at the electrodes over the lesion (BM 14-18) with secondary spreading to the frontobasal cortex (BM 1-3); they were continuous at the start of the episode and progressed towards a discontinuous pattern, consistent with burst-suppression, over 1 to 2 minutes (figure 4). Eight clusters of seizures were recorded with the same clinical characteristics as observed by surface video-EEG recording. A fast discharge followed by a sharp-wave rhythmic activity on BM 14-18 over the lesion, lasting 50 seconds, preceded the first clinical event. The ictal EEG pattern consisted of a fast activity on BM 14-18, spreading to nearby electrodes and lasting for one second, followed by a slow-wave component at the same sites. On BM 14-18, however, the fast activity lasted longer (3 seconds). The clinical manifestations occurred during the slow component. Unfortunately, the motor cortex was not investigated. The second clinical event occurred 32 seconds after the first. There was a widespread fast activity, also at the temporal electrodes, followed by a slow component; the clinical manifestations were associated with the slow component. The subsequent events during the cluster were longer but no anatomo-electroclinical correlation was possible due to the sampling bias of SEEG. At the beginning of the cluster, the focal discharge persisted between spasms and then became discontinuous; when the spasm became tonic, the focal discharge disappeared (figure 5).
The patient underwent left anterior frontal cortectomy, corresponding to the external BM area, in December 2007 (neurosurgeon: GD), at the age of 16 years. Anatomo-pathological analysis revealed no dysplasia or ischaemia, but hyaline astrocytic inclusions were found which were identified to be PAS negative but strongly positive for filamin A, by immunocytochemical analysis.
The postoperative outcome was favourable with only three seizures during the three years of follow-up (Engel class II). The patient's performance at school improved and she was able to begin professional education. Her neuropsychological evaluation remained stable (WISC-III: TIQ: 65) with a dysexecutive frontal syndrome.
Discussion
This case presented here illustrates how late-onset isolated epileptic spasms may have a focal origin and be cured by adapted cortical resection.
The patient's epileptic episodes corresponded to the definition of Epileptic Spasms (Vigevano et al., 2001) because the duration of muscular contraction was intermediate between myoclonic movement and tonic seizure. During the cluster, there was a progression from brief and symmetric spasms to tonic asymmetric spasms. The EEG characteristic of the spasms was a diffuse high-voltage, slow-wave complex; when the spasm became tonic and asymmetric, there was a period of symmetric or asymmetric voltage attenuation following the slow-wave pattern. This electroclinical pattern is common in ES (Vigevano et al., 2007).
Using the ILAE classification group, this case of ES corresponds to generalised-onset seizures and to focal-onset secondary generalised seizures, although the focal-onset seizure was noted as “unverified” (Engel, 2006). When the ES occurred in an isolated manner it was difficult to determine the origin. Our patient presented with no partial seizures and no clear focal scalp EEG anomalies, suggestive of focal epilepsy. This was concordant with the cases of cryptogenic late-onset spasms reported by Eisermann et al. (2006) which evoked an epileptic encephalopathy intermediate between West syndrome and Lennox-Gastaut syndrome. A focal onset was nevertheless suspected in our case because FDG-TEP-MRI co-recording showed a localised area of hypometabolism over the medial part of the left superior frontal gyrus, consistent with scalp EEG data. This allowed us to guide implantation of depth electrodes. SEEG showed a clinically silent focal discharge lasting 50 seconds preceding the spasms in the hypometabolism area that was not detected by surface EEG. The first spasm corresponded to fast activity in this area, then, during the cluster there was a widespread fast activity followed by a slow component during spasms. Resection of this area involved in the focal discharge area was associated with a good seizure outcome. Similar data have been obtained from electrocorticography studies showing the dynamic electrocorticographic changes associated with ES; in cases in which a focal-onset seizure zone was identified, based on high frequency oscillations (HFOs) generated by the neocortex and the subsequent propagation to the sensory motor cortex associated with body jerking, surgical resection of the cortical region generating ictal HFOs was associated with a good surgical outcome (RamachandranNair et al., 2008; Nariai et al., 2011). In our case, we can speculate that if there had been depth electrode contacts within the motor cortex, the longer lasting, more tonic seizures would most likely have been shown to be the result of spread of fast activity into this region; the slow waves occurring together with these clinical events were most likely not the primary electrical event corresponding to clinical expression.
Moreover, the neuropathological findings in our case were unusual. Similar astrocytic inclusions, immuno-positive for filamin A (an actin-binding protein involved in neuronal migration), have been identified in cases of Aicardi syndrome (Van den Veyver et al., 2004) and in some surgical resections from paediatric epileptic patients (Hazrati et al., 2008) presenting with seizures in their first year of life and mild-to-moderate developmental delay. The clinical and pathophysiological significance of these inclusions remains unknown.
Finally, late-onset ES may be a common manifestation with variable cortical, often frontal, trigger zones. Therefore, in cases of late-onset ES, investigations should include a search for a focal trigger zone, even when scalp EEG abnormalities are not clearly localised. SEEG or electrocorticography should be used to seek any focal rapid discharges preceding spasms; FDG-PET-MRI co-recording should be considered for guiding intracranial investigation.
Acknowledgments
We are grateful to Dr Jean-Yves Tanguy for performing MRI and to Dr Francine Chassoux for her precious collaboration in interpreting MRI-FDG-PET data.
Disclosures
None of the authors has any conflict of interest to disclose.
Legend for video sequence At 22:22:53, a noise awoke the patient; a discharge of slow spike-and-waves appeared in a bilateral frontotemporal area, becoming faster and localised in left temporal area. At 22:23:53, she had a first spasm involving a sudden head flexion with adduction of upper limbs and lower limb flexion, lasting 0.5 seconds. These motor sequences were repeated (3-4 every 20 seconds) and were short and symmetric. At 22:27, they became asymmetric, the abduction movement being broader, more tonic on right limbs, and longer (lasting 2 seconds), and the patient had a hiccup. Consciousness was preserved and language was normal. The spasms stopped at 22:39, such that the cluster lasted 16 minutes. During the first events, EEG revealed a diffuse high-voltage sharp-and-slow-wave complex. The spasms then became asymmetric and more tonic, with a period of voltage attenuation following the slow-wave pattern. During the asymmetric spasm, the period of voltage attenuation tended to be diffuse, symmetric or asymmetric, longer and more evident over left central and temporal regions. The localisation of the slow-wave pattern was diffuse with a maximum peak over the vertex region. Key words for video research on www.epilepticdisorders.com Syndrome: focal non-idiopathic frontal (FLE) Etiology: brain malformation (not specified) Phenomenology: spasm (epileptic); tonic posture; axial Localization: frontal lobe (left)