Epileptic Disorders
MENUIctal spitting in non-dominant temporal lobe epilepsy: an anatomo-electrophysiological correlation Volume 20, issue 2, April 2018
Case study
A 28-year-old, right-handed woman with a history of medically refractory epilepsy with focal seizures since the age of 17 was referred to our centre for presurgical evaluation. Her previous medical history was unremarkable, as well as the neurological examination. She was taking zonisamide, 300 mg BID, and eslicarbazepine acetate, 800 mg/day, but previously was on different AED combinations.
The patient presented three types of seizure semiology. Type 1 was focal cognitive (complex partial) seizures characterized by an uprising epigastric aura, intense feeling of fear with a scared face, associated with complex visual hallucinations in the form of facial deformation (prosopometamorphopsia), left-hand dystonia, and oral automatisms, followed by subtle loss of contact with ictal speech (“I spit at god's feet” in her native language), and then by ictal spitting and postictal confusion. Seizure duration lasted for one to two minutes with a frequency of one to two per month. Type 2 was focal autonomic (simple partial) seizures with awareness, epigastric aura and oral automatisms with a frequency of one per month. This form of seizure occurred mainly during the night. Type 3 was focal to bilateral tonic-clonic (secondary generalized) seizures during sleep and presented with epigastric aura, followed by a head deviation to the left with tonic-clonic evolution with a frequency of one to two per year. Factors that appeared to facilitate seizures included menstrual periods and sleep deprivation.
Non-invasive work-up
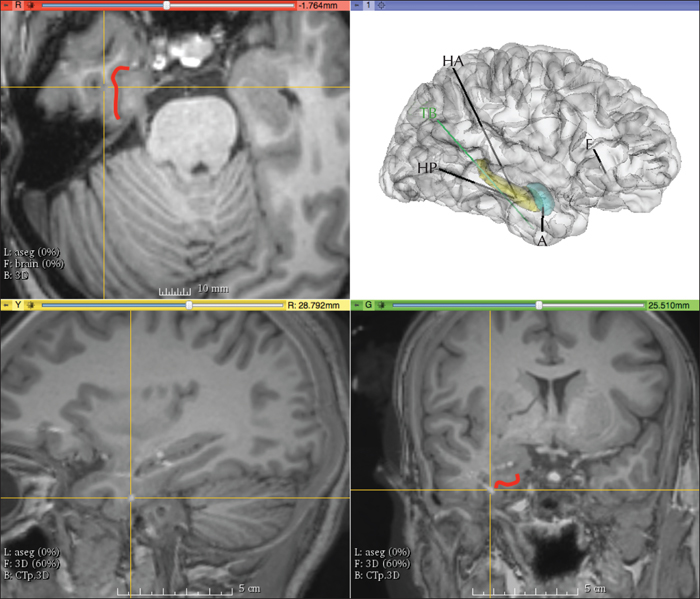
VEEG monitoring showed normal background with interictal activity in the right anterior temporal region (maximum in T2-T4). Ten clinical seizures were recorded during two VEEG sessions (four autonomic, five cognitive, and one focal to bilateral tonic-clonic seizure) with the above-described semiology, suggestive of an ictal onset zone in the right anterior and medial temporal lobe. EEG onset occurred at the right anterior temporal region (maximum in T2-T4) with delta frequencies at 2.5-3 Hz, with propagation after seven seconds to the left hemisphere. VEEG monitoring was suggestive of right temporal lobe epilepsy. Functional neuroimaging (ictal-interictal SPECT and SISCOM) concurred with anterior right temporal lobe epilepsy, mainly related to hyperperfusion at medial and anterior temporal regions. 3-Tesla brain MRI showed marginal amygdala asymmetry in the absence of any other sign or lesion (supplementary figure 1). The neuropsychological evaluation was hindered by cultural and language constraints. Nevertheless, global attention impairment, visual-verbal memory, and dysexecutive deficits were detected.
Hypothesis 1
Seizure semiology together with indirect MRI data (increased size of right amygdala in the absence of any other lesion) and VEEG findings suggested a possible epileptogenic zone in the right anterior temporal lobe.
Invasive procedures
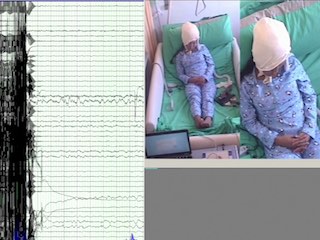
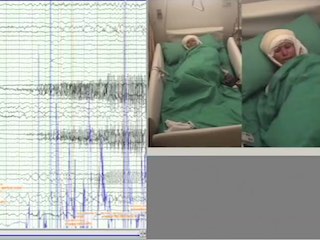
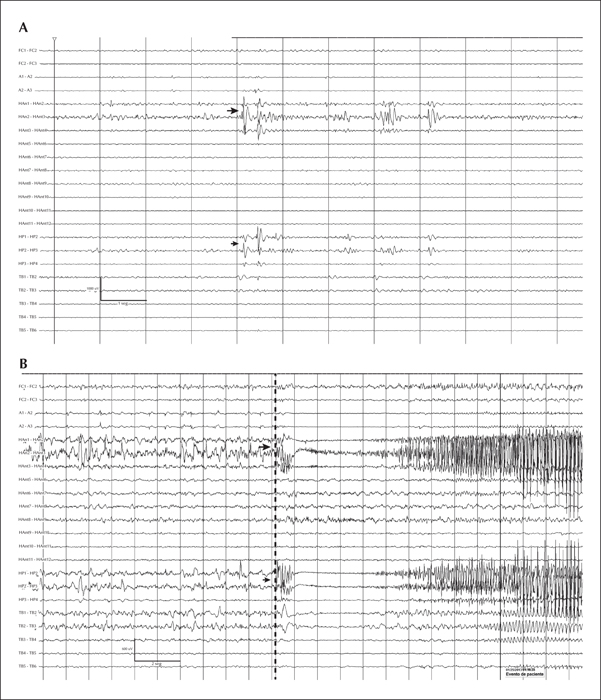
Stereo-electroencephalography (SEEG) for intracranial evaluation was indicated in order to identify the epileptogenic zone and better define a possible surgical resection limit (figure 1). During the two weeks of monitoring interictal activity, polyspike-waves at electrodes at the right hippocampus were registered (figure 2A). Eight clinical and two sub-clinical seizures were also recorded; in six of the clinical seizures, ictal spitting was present. All the seizures were characterized by rhythmic spike-slow waves with a frequency of 5-7 Hz, maximum at the right hippocampus head and temporobasal regions (figure 2B). The average duration of spitting was 70.66 seconds (standard deviation: 23.14 seconds) and the mean duration of such seizures was 226.25 seconds (standard deviation: 78.59 seconds). In all seizures, oral and manual automatisms preceded ictal spitting (video 1). It is of note that, after direct electrical stimulation (DES) (50-Hz; 5-second trains; 1 to 3-mA) to the right basal temporal and entorhinal cortex (EC), drooling and posterior spitting were evoked without triggering seizures or post-discharges (video 2). Spitting was consistently evoked upon another DES pulse. To the best of our knowledge, this is the first case in which spitting has been triggered by DES.
Actions taken
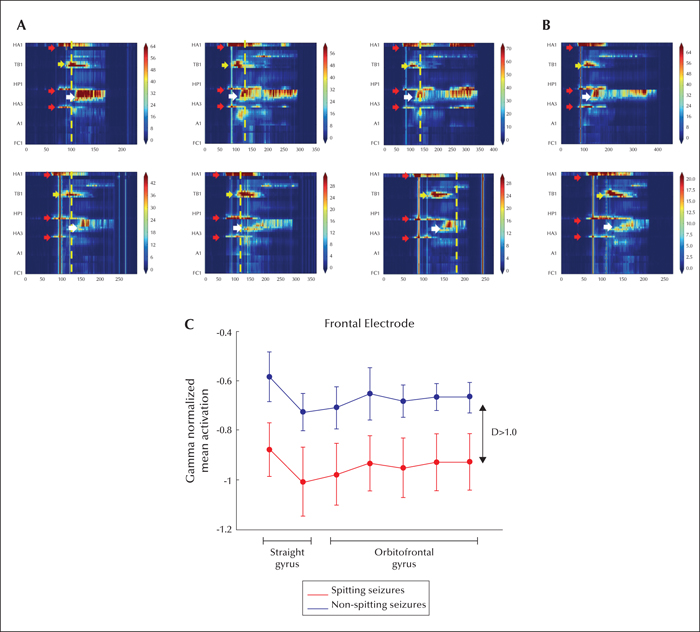
A custom-made code for the analysis of the EEG signal power spectrum was developed using Python (Vila-Vidal et al., 2017) to describe the time-varying spectral activity of each recorded channel during seizures. The time at seizure onset and termination were independently recorded by two epileptologists (RR and AP). For each seizure, SEEG recordings during the marked ictal epoch, together with 60 seconds of pre-ictal and 60 seconds of postictal epochs, were evaluated. The analysis was performed as follows: first, the instantaneous power of each channel in the monopolar referencing form was computed with the Hilbert transform across distinct narrow frequency bands (range: 3-160 Hz). Channel activation during ictal epochs was then assessed by normalizing the instantaneous power to a baseline distribution of values obtained from the initial 40 seconds of selected pre-ictal epoch (figures 3A, B). As shown in figure 3, there was a typical pattern of spectral changes for all eight clinical seizures that included: the earliest activation at deepest contacts from the right anterior hippocampus (seizure onset zone); a later activation of the right temporobasal cortex (comprising the EC), acting as an early propagation zone; and finally, propagation to the more lateral contacts of the anterior temporal lobe (figure 3A). The entire pattern of activation included the site of initiation and organization of seizures corresponding to the epileptogenic zone.
As shown in the broadband (3-160 Hz) spectral analysis, ictal spitting occurred after the activation of the EC and once the contacts at the anterior hippocampus were recruited. In particular, no specific broadband differences between seizures, with or without spitting, were found (figure 3A and 3B). However, when comparing the gamma-band (20-70-Hz) activation of frontal channels during ictal epochs (Vila-Vidal et al., 2017), a significant difference (confidence interval; level=0.1) with large effect size (D>1; Cohen's D) arose between ictal spitting and non-spitting seizures (figure 3C) (Cohen, 1992).
Follow-up
Based on the anatomo-electroclinical correlations registered by SEEG, a right anterior temporal lobectomy was performed. Neuropathological examination revealed focal cortical dysplasia type Ia (FCD-Ia) which involved the amygdala and temporobasal region, but not the hippocampal formation. All AEDs were withdrawn for more than one year.
Discussion
Ictal spitting is an extremely rare vegetative automatism and has been described in only 0.3% of all patients undergoing long-term VEEG monitoring, and in 1.03% of subjects with TLE (Musilová et al., 2010). The Bancaud group intensively studied gustatory auras and found that two out of 718 patients presented this semiology (Hausser-Hauw and Bancaud, 1987). In the largest review so far, Voss et al. evaluated 2,500 patients and found five with ictal spitting, all with right TLE. Indeed, the origin in most of the cases has been related to the non-dominant mesial temporal lobe (Voss et al., 1999; Kellinghaus et al., 2003; Park et al., 2007), but few have been reported with a possible origin in the dominant temporal, insular, or frontal lobes (Clemens et al., 2005; Caboclo et al., 2006; Janszky et al., 2007; Vojvodic et al., 2013).
In the case described here, ictal spitting occurred during ictal speech (cursing) with religious content in the patient's native language and was associated with visual face hallucinations (prosopometamorphopsia). Since spitting can be a part of a sacred ritual, associated with a particular culture, it might form part of a complex, religion-related semiology that is more frequent in non-dominant TLE (Özkara et al., 2004; Vural et al., 2015). Probably, the physiopathology of ictal spitting shares mechanisms with other oroalimentary automatisms in which a primitive cortical reflex is released (Loddenkemper and Kotagal, 2005). At the same time, prosopometamorphopsia is another extremely rare seizure semiology and can be considered a particular form of metamorphopsia. Bien et al. described that complex visual hallucinations are concordant with an anteromedial temporal localization (Bien et al., 2000). Specifically, face deformation has been localized by ictal SPECT at the right temporo-occipital junction (Heo et al., 2004).
On the other hand, recent investigations have revealed that EC seems to encode current contexts used posteriorly by the hippocampus to create individual representations from this information (Jacobs et al., 2010). In our case, therefore, abnormal activation of the EC could have caused visual information to be erroneously encoded, leading to the automatism of spitting after the propagation to cortico-subcortical structures. As proposed by Kahane and Voss, ictal spitting is related to an autonomic response upon stimulation of the limbic system (Voss et al., 1999; Kahane and Minotti, 2000). This is supported by the fact that in most of the seizures, the patient spat after oral automatisms with no other complex behaviour, and that DES to the EC evoked spitting without triggering ictal activity or post-discharges on SEEG. However, this finding should be interpreted with caution. As the spectral analysis revealed (figure 3), the activation of the EC is a necessary, but not a sufficient, condition for ictal spitting to occur. In other words, the EC behaves as an activation node of a network that includes distant structures in which the complex automatism might be encoded. In this regard, we showed that gamma-band neural activity in the frontal lobe could discriminate between seizures with and without ictal spitting (figure 3C). Therefore, our analysis reveals that the basal medial temporal region including the EC is the structure where ictal spitting is triggered but not encoded, as verified using DES and spectral analysis.
Conclusion
Ictal spitting is a very uncommon type of seizure semiology associated predominately with non-dominant TLE. As we have described here, the basal medial temporal region including the EC is probably a necessary propagated region for the generation of this complex autonomic behaviour. The acquired knowledge of this electroclinical correlation may improve our understanding of the neural substrates of this uncommon semiology.
Supplementary data
Supplementary figure is available on the www.epilepticdisorders.com website.
Disclosures
None of the authors have any conflict of interest to declare. M. Quevedo-Diaz is financially supported by the National Council of Science and Technology (CONACyT), Mexico City, Mexico.