Epileptic Disorders
MENUEpileptic nystagmus Volume 8, issue 4, December 2006
Auteur(s) : Pinar Bekdik, Ufuk Sener, Irem Fatma Asan, Murat Ozcelik, Yasar Zorlu
Tepecik Training Hospital, Izmir, Turkey
Article reçu le 12 Septembre 2005, accepté le 2 Août 2006
Epileptic nystagmus (EN) is a rare ictal phenomenon characterized by rapid, repetitive eye movements and caused by epileptic activity (Kaplan and Tusa, 1993, Yılmaz et al., 2004). It may be the only symptom of an epileptic seizure. In the majority of reported cases, the nystagmus is jerky, horizontal, conjugate, and usually accompanied by tonic, horizontal eye and head deviations. Typically, the seizure focus is in the temporo-parieto-occipital region of the cerebral hemisphere, contralateral to the direction of the nystagmus beats, and in some cases, the nystagmus may result from excitation of the cortical centres for pursuit or optokinetic eye movements (Kaplan and Tusa, 1993). We report on a case of epileptic nystagmus and discuss the clinical and EEG features of this rare ictal symptom.Case report
A 19-year-old, right-handed female patient was being treated for partial epilepsy. Her epileptic seizures began at the age of 7. The seizures were defined by an odd odor, feelings of fatigue, vertigo, nausea, a burning feeling on the neck and head, and rare, secondary generalization. Following her first secondary generalized tonic-clonic seizure at the age of 7, she was diagnosed with epilepsy, and phenobarbital (PB) treatment was initiated. Carbamazepine (CBZ) was added to her treatment because of the persistence of 3-4 seizures per month during the phenobarbital treatment. Seizure frequency was two per month under this treatment. We started following the patient at our “intractable epilepsy clinic” in 2002. Her treatment was changed to sodium valproate and carbamazepine as only limited seizure control was being achieved with the previous combination. Her seizure frequency was 3-4 per year whilst on this treatment. The patient’s compliance with the treatment was not ideal, and nearly all the seizures occurred during the periods the patient was not in compliance with her medications; drug serum levels were subtherapeutic during such periods. In May 2005, after five, secondary generalized tonic-clonic seizures in 24 hours, the patient was admitted to our clinic for her treatment to be properly adjusted.Prenatal history was unremarkable apart from doses of 500 mg metamizol sodium taken by the mother at seven months of gestation for analgesic purposes. Motor and mental development was normal. She experienced a febrile convulsion at the age of three years. The history revealed a fall from 1.5 meters at the age of six, which healed without treatment. There was no family history of febrile convulsions and/or epilepsy.
Clinical examination was normal. No abnormal findings, apart from post-ictal confusion, were found at her first neurological examination, and repetitive neurological examinations were normal. Routine haematology and biochemistry examinations revealed no abnormal findings. Serum VPA (18 μgr / dl) and CBZ levels (1.3 μg/dL), measured a day after admission to our clinic, were found to be low.
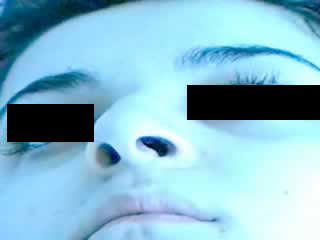
EEG on admission revealed active epileptic activity in the temporal region of the right hemisphere (T4-T6) (( figure 1 )A-B). No pathological findings, apart from frontotemporal atrophy, were present on brain computerized tomography (CT) and magnetic resonance imaging (MRI) (( figure 2 )). Considering that the seizures were caused by drug cessation, her initial antiepileptic drug treatment (VPA + CBZ) was started again. On the 5th day from admission, a seizure, characterized by a deviation of the head and eyes to the left, and a left-beating nystagmus, was observed. During this episode, the eye movements did not cross the midline. The patient was awake during the seizure and she could give meaningful, but one-word answers to questions (video sequence). Intravenous midazolam was administered because of the increased seizure duration (> 30 minutes), and the seizure ended. On the same day, a second EEG revealed an epileptic area on the temporal region of the right hemisphere.
Discussion
Epileptic Nystagmus is a rare type of eye movement occurring during an epileptic seizure (Gire et al., 2001, Yılmaz et al., 2004). Salanova et al. observed nystagmoid eye movements in less than 10% of the cases with occipital lobe epilepsy (Salanova et al., 1992). EN is horizontal and conjugated in the majority of cases (Kaplan and Tusa, 1993), but monocular nystagmus has also been reported as an ictal sign (Grant et al., 2002). As in the present case, tonic, horizontal eye and head deviations are frequently observed, and are accompanied by EN (Harris et al., 1997, Garcia-Pastor, 2002, Pfefferkorn et al., 2004).Even though the mechanism of EN is not well known, it has been associated with the stimulation of the cortical saccade, or the pursuit or optokinetic eye movement centres (Kaplan and Tusa, 1993, Yılmaz et al., 2004). Characteristics of the conjugated horizontal EN differ as a function of the cortical region it originates from. EN originating from the temporo-occipital or frontal cortex regions is associated with saccadic eye movements, the rapid phase of nystagmus being directed away from the seizure focus (Kaplan and Tusa, 1993, Yılmaz et al., 2004). Each contraversive, quick-phase eye movement induced by the seizure discharge is followed by a slow phase that brings the eyes toward the midline of the orbit. The slow phase drift caused by the leaky neural integrator, has a characteristic velocity-decreasing profile, i.e. the speed of the eye movements slows down as the eye approaches the midline of the orbit. However, the eyes never cross the midline of the orbit (Kaplan and Tusa, 1993). In EN originating from the temporo-occipital cortex region associated with pursuit eye movements, each ipsiversive, slow-phase eye movement induced by the seizure discharge is followed by a reflexive, quick phase as the eye reaches the far eccentric position in the orbit (Kaplan and Tusa, 1993, Tusa et al., 1990). Unlike EN induced by stimulating saccade regions, EN induced by stimulating pursuit regions results in linear, slow phases in which the eye movements are likely to cross the midline (Kaplan and Tusa, 1993). In EN originating from cortical regions associated with optokinetic eye movements, the type and lateralization of EN is similar to the ones observed in nystagmus arising from cortical region associated with pursuit eye movements (Kaplan and Tusa, 1993).
EN may occur at all ages. Harris et al. reported the case of an infant in whom EN was first noted at 10 days of age (Harris et al., 1997). He suggested that the EN had a cortical origin; infant cortex has functioning efferent connections to brainstem oculomotor centres (Harris et al., 1997). The cortical origin and clinical characteristics of the epileptic discharges which cause EN in children are the same as those seen in adults (Kaplan and Tusa, 1993, Tusa et al., 1990).
In our case, we observed a horizontal nystagmus with deviation of the head and eyes to the left. The fast phase of the nystagmus was towards the left, and the slow phase did not go beyond the midline. These features of nystagmus led to the consideration that epileptic activity had arisen from the right temporo-occipital cortex region associated with saccadic eye movements. Consistent with this clinical localization, EEG showed an epileptic activity in the right temporal region.
Epileptic Nystagmus arising from cortical saccade centres localized in temporo-parietooccipital regions can occur both in awake and comatosed patients. In comatosed patients, the slow phase of this type of nystagmus can be explained by an altered mental status disturbing the gaze-holding system (leaky neural integrator). In addition to coma, a number of substances can cause a leaky neural integrator, including alcohol, sedatives and antiepileptic medications (Kaplan and Tusa, 1993). Our patient was awake but on antiepileptic medication.
In addition to comatosed and awake patients, EN has also been reported in patients during nonconvulsive status epilepticus (Yilmaz et al., 2004). In these patients, ictal recording is very important in order to describe and classify the phenomenon. Unfortunately, we were unable to perform ictal recording since the seizure was not repeated during EEG recording. In our patient, the EN lasted more than 30 minutes, and after the administration of intravenous midazolam, cessation of nystagmus was observed. For this reason, we think that the EN was an isolated ictal finding of nonconvulsive status epilepticus even though no simultaneous EEG recording was performed.
The majority of the cases reported in the literature present a structural CNS lesion in the central nervous system (Yılmaz et al., 2004, Kaplan and Tusa, 1993). In our case however, MRI revealed only cortical atrophy. Clinical and EEG findings in our case suggest that EN, an ictal phenomenon that can be easily overlooked, is important in the lateralization of epileptic activity.