Epileptic Disorders
MENUEffectiveness and safety of perampanel in Chinese paediatric patients (2-14 years) with refractory epilepsy: a retrospective, observational study Volume 23, issue 6, December 2021
Epilepsy is a common neurological disease in children and adolescents. The incidence of epilepsy in children ranges from 41-187/100,000 and the prevalence of epilepsy ranges from 3.6 to 4.4 per 1000 children in developing countries [1]. Of children with epilepsy, about 10% have drug-resistant epilepsy [2]. Drug-resistant epilepsy represents a significant burden for patients and can reduce quality of life. Perampanel is a structurally novel, selective, non-competitive α-amino-3-hydroxy -5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptor antagonist that functions by blocking glutamate activity in the postsynaptic AMPA receptors [3].
Perampanel is a third-generation anti-seizure medication (ASM) used for focal seizures (FS), with or without focal to bilateral tonic–clonic seizures (FBTCS), and generalized tonic–clonic seizures (GTCS) in patients with epilepsy aged 12 years and above in multiple countries around the world. In 2018, perampanel was approved by the US Food and Drug Administration for the treatment of FS (as adjunctive therapy and monotherapy) in patients aged four years and older [4]. Perampanel entered the Chinese market and was approved to treat FS, with or without FBTCS, and GTCS in patients aged 12 years and older in September 2019.
Study 232 (NCT01527006) was the first study to evaluate the pharmacokinetics and preliminary tolerability and effectiveness of adjunctive perampanel in epilepsy patients aged 2-12 years [5]. Study 311 (NCT02849626) was a multi-centre, open-label, single-arm study in paediatric patients, aged 4-12 years, with FS (with/without FBTCS) or GTCS [6]. Notably, early prescription of third-generation ASMs as add-on treatment may potentially become a new strategy for people with epilepsy, increasing the tolerability and effectiveness of the drugs used [7]. Rohracher et al. [8] found that patients using co-administered enzyme-inducing anti-seizure drugs (EIASDs, such as carbamazepine, oxcarbazepine, phenytoin, and primidone) appeared to have a lower likelihood of seizure freedom than those using co-administered non-EIASDs. Use of co-administrated synaptic vesicle protein 2A (SV2A) modulators was associated with a higher chance of seizure freedom. Perampanel's apparent oral clearance is increased by EIASDs and perampanel exposure is thereby lowered, therefore, a higher perampanel dose and more frequent up-titration is needed for patients receiving perampanel concomitantly with EIASDs to achieve a level of effectiveness similar to that in patients receiving non-EIASDs [6, 9, 10]. A previous study also suggested that the serum perampanel concentration begins to increase from one week after discontinuation of carbamazepine, and thereafter continues to increase for eight weeks [11]. A sub-analysis of Study 231 and an extension of Study 233 investigated drug–drug interactions between perampanel and concomitantly used EIASDs [12]. Levetiracetam and brivaracetam are both modulators of SV2A-mediated neurotransmitter release [13, 14], but in China, levetiracetam is currently the only SV2A modulator available, as brivaracetam has not been approved for use in China. Oxcarbazepine is the most commonly used EIASD in paediatric patients.
Clinical data on the safety and effectiveness of perampanel in real-world clinical settings involving Chinese children are limited, as perampanel has only recently been approved for use in China. In this study, we provide a real-world evaluation of the effectiveness and safety of perampanel use in Chinese mainland paediatric neurology clinics. We describe our experience with perampanel as adjunctive treatment in patients with refractory epilepsy, aged 2-14 years, and relate the drug-drug interactions between perampanel and concomitantly used oxcarbazepine or levetiracetam.
Materials and methods
Patients
This retrospective observational study was performed between September 2019 and September 2020 in the Department of Paediatric Neurology, the Children's Hospital of Soochow University and the Affiliated Hospital of Xuzhou Medical University. Inclusion criteria were age 2-14 years, history of refractory epilepsy; and follow-up with ≥six months of perampanel as add-on therapy.
The institutional review board of each study centre approved the study, and all patients and their parents provided written informed consent before participation. Since perampanel is only approved by the Chinese Food and Drug Administration for the treatment of seizures in patients aged 12 years above, informed consent was also obtained for off-label use of perampanel when the patient was under 12 years old. Patients were stratified by age (2-7 years; 7-14 years) and according to the presence or absence of concomitant oxcarbazepine and levetiracetam.
Collected data
Data are presented for total patients and by age of cohort (younger [2-7 years] vs older [7-14 years] patients), and for patients with/without concomitant oxcarbazepine, and with/without concomitant levetiracetam treatment. The following data were collected at baseline: family and personal medical histories, age at epilepsy onset, age at perampanel initiation, seizure and epilepsy type, epilepsy aetiology, seizure frequency, and most common concomitant anti-epileptic drugs (AEDs). Seizure types and epilepsy syndromes were classified in accordance with the International League Against Epilepsy.
Patients received perampanel at doses ranging from 1–2 mg/day to a maximum of 12 mg/day given once-daily at bedtime. Perampanel was administered at a once-daily dose of 2 mg/day in patients aged seven years and above. Up-titration was usually performed by increments of 1–2 mg every 3–4 weeks. However, in children aged from 2 to <7 years, smaller increments (1 mg) were applied and up-titration was slower (1 mg every 3–4 weeks). The perampanel titration schedule was at the discretion of the treating physician, according to the medical needs of the patient. If a patient did not tolerate up-titrated treatment, the dose could be reduced to the previous level. Treatment was discontinued when the neurologist considered that perampanel was not effective or in cases in which seizure aggravation or intolerable side effects were suspected.
The primary endpoint was safety and tolerability of perampanel. Safety assessments included the incidence of adverse effects (AEs) and serious adverse events (SAEs). Efficacy endpoints were the proportion of patients who were seizure-free, the proportion of responders (patients with ≥ 50% seizure reduction from baseline), and the retention rate on perampanel at six and 12 months. Seizure freedom at 12 months was defined as no seizures during the previous six months, whereas seizure freedom at six months was defined as no seizures for three months prior to the visit. Seizure reduction measures were based on monthly seizure frequency. The safety endpoints included the proportion of patients with AEs at six and 12 months, and the proportion of patients with AEs that led to discontinuation of perampanel at six and 12 months. AEs were gleaned from clinical records, and were classified by study investigators as mild, moderate, or severe. Only AEs considered by the participating physicians to be related to perampanel were included in the analysis. Efficacy and safety outcomes were also assessed by age, and with/without oxcarbazepine or levetiracetam use.
Data analysis
Continuous variables are presented as mean ± standard deviation (SD). Categorical variables were summarized as frequency and percentage. The chi-square test or Fisher's exact test was used, as appropriate, for analysis of between-group differences in discrete variables. Values of p < 0.05 were defined as statistically significant. Statistical analyses were performed using SPSS version 25 software (IBM, New York, NY).
Results
Demographic and baseline characteristics of patients
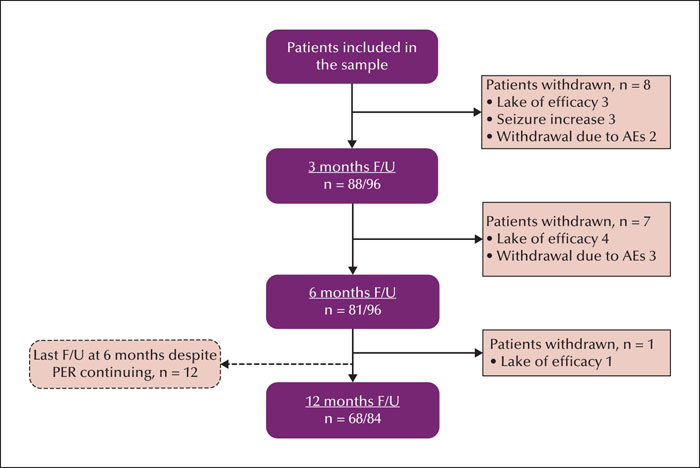
Ninety-six paediatric patients who experienced pharmacoresistant epilepsy and who received treatment with perampanel as adjunctive therapy were included in the analysis. All patients had complete data for ≥ six months of follow-up which was available for analysis. A flowchart of patient numbers and retention rates at the 6- and 12-month follow-up time points is presented in figure 1. In total, 96 paediatric patients were included in the analysis (43 females; aged 2-14 years, mean: 7.9 ± 3.2 years). Sixty-two patients (64.6%) had FS, and 20 patients (20.8%) had GTCS. There were 44 patients (45.8%) aged 2-7 years (mean: 4.8 ± 1.1 years) and 52 patients (54.2%) aged 7-14 years (mean: 10.5 ± 1.8 years). Thirty-seven patients (38.5%) were taking concomitant oxcarbazepine and 48 patients (50.0%) were taking concomitant levetiracetam. Patient demographics and baseline clinical characteristics are provided in table 1. Age at perampanel initiation and sex distribution were not significantly different between the oxcarbazepine/levetiracetam cohort vs the non-oxcarbazepine/levetiracetam cohort.
Perampanel dose and treatment
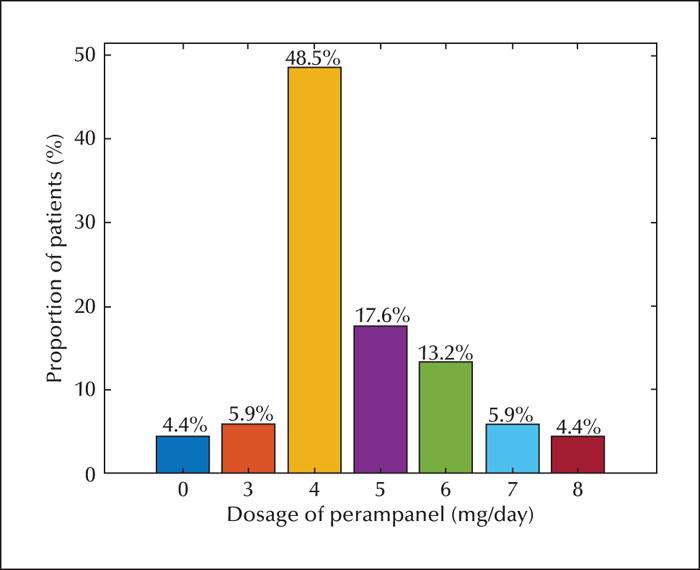
At the end of 12 months of follow-up, 68 patients (70.8%) continued with perampanel. The mean ± SD maximal daily dose of perampanel for all patients was 4.7 ± 1.3 mg/day. The most common dose was 4 mg (48.5% of 68 patients) (figure 2). This was similar across the levetiracetam cohort, but the mean maximal value of perampanel was slightly higher in the older patients (5.1 ± 1.4 mg/day) than in the younger patients (4.1 ± 0.96 mg/day). Patients with concomitant oxcarbazepine use (4.8 ± 1.2 mg/day) required a higher dosage of perampanel than subjects who did not use oxcarbazepine (4.5 ± 1.4 mg/day) (table 1). However, these differences in perampanel dosage between patients in different age cohorts or with/without oxcarbazepine were not statistically significant.
The most common concomitant AEDs taken by paediatric patients with refractory epilepsy were levetiracetam, oxcarbazepine, lamotrigine, valproic acid and topiramate. Differences in concomitant AEDs, including lamotrigine, valproic acid and topiramate, taken by patients in different age cohorts or with/without oxcarbazepine/levetiracetam were not statistically significant. The proportion of patients with concomitant levetiracetam was not significantly different between the oxcarbazepine cohort vs the non-oxcarbazepine cohort. Also, the proportion of patients taking concomitant oxcarbazepine was similar between patients with and without levetiracetam.
Effectiveness of perampanel at six and 12 months of follow-up
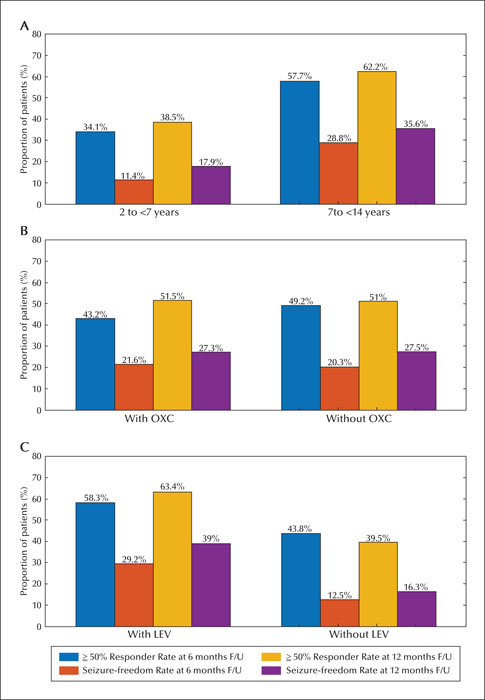
We were able to review 81 and 68 patients who remained on perampanel at six and 12 months, respectively. The retention rates for perampanel treatment were 84.4% (six months) and 81.0% (12 months). Fifteen patients discontinued perampanel within the first six months and one patient discontinued the drug between six and 12 months (figure 1). The reasons for discontinuation of perampanel were lack of effectiveness in 8.3% (n = 8), intolerable AEs in 5.2% (n = 5), and aggravation of seizure in 3.1% (n = 3) of patients. A summary of perampanel effectiveness at the six- and 12-month follow-up time points is presented in figure 3. With perampanel treatment, seizure reduction varied over time, with 46.9% (45/96) and 51.2% (43/84) of patients showing a ≥50% response rate after six months and 12 months of follow-up, respectively. The number of patients experiencing complete seizure control increased from 20 (20.8%) at six months to 23 (27.4%) at 12 months. Nineteen patients reported periods of seizure freedom lasting ≥six months at the final follow-up visit, and one patient gained seizure freedom at six months, but lost this status by the 12-month follow-up visit.
The 50% response rates were 34.1% and 57.7% for the younger and older cohorts after six months of follow-up (p = 0.037) and 38.5% and 62.2% after 12 months of follow-up (p = 0.030), respectively. The proportion of patients with seizure freedom was higher in the patients aged 7-14 years than in those aged 2-7 years at both the six-month (28.8% vs 11.4%, p = 0.037) and the 12-month (35.6% vs 17.9%, p = 0.071) follow-up time points (figure 3A). Significant differences were observed in responder rates for patients in the different age cohorts, besides seizure freedom, after 12 months of follow-up.
There was a similar response between patients who received oxcarbazepine and those who did not receive oxcarbazepine (figure 3B). The efficacy of perampanel varied according to the presence or absence of concomitant levetiracetam. The 50% response rates were 58.3% and 43.8% for patients with and without concomitant levetiracetam, respectively, after six months of follow-up (p = 0.032), and 63.4% and 39.5%, respectively, after 12 months of follow-up (p = 0.029). The proportion of patients with seizure freedom was higher in patients with concomitant levetiracetam than in those without at the six-month (29.2% vs 12.5%, p = 0.044) and 12-month (39.0% vs 16.3%, p = 0.019) follow-up time points (figure 3C).
Safety and tolerability
Overall, 22.9% of patients (22/96) had experienced at least one AE. These were mild or moderate in most patients, thus no SAEs were reported. Most AEs occurred within the first 3–6 months, with a few additional AEs occurring over the remaining follow-up time (table 2). All AEs improved when perampanel dosage was tapered or discontinued. The most frequent AEs were irritability (12 patients, 12.5%), somnolence (nine patients, 9.4%), and dizziness (seven patients, 7.3%). Overall, 5.2% of patients discontinued perampanel due to AEs. Headache and skin allergy were both reported in five patients (5.2%). Ataxia was seen in four patients (4.2%). Aggression and fatigue were reported in three patients (3.1%). Sleep disorders (i.e., insomnia) and poor appetite were each noted in one patient (1.0%). AEs were more common in older patients (30.8%) than younger patients (13.6%) (p = 0.047). Overall, four patients (7.7%) in the older cohort discontinued due to AEs, whereas only one patient (2.3%) in the younger cohort discontinued because of AEs. AEs were more common in older than in younger children.
No significant difference in AEs was seen between patients with and without oxcarbazepine (22.0% and 24.3% of patients, respectively). Levetiracetam has previously been associated with psychiatric AEs and is known to induce aggressiveness. In this study, aggression was present in two patients without concomitant levetiracetam and in one patient with concomitant levetiracetam. AEs were more common in patients without concomitant levetiracetam compared to those with concomitant levetiracetam (27.0% vs 18.8%), although this difference was not statistically significant.
Discussion
In this study, we describe our experience with perampanel in Chinese paediatric patients. We designed the study to clarify whether age- and medication-related factors affect the clinical response and risk of AEs in patients treated with adjunctive perampanel. Few studies have addressed this issue. Our retrospective data analysis demonstrated that perampanel administered as an adjunctive treatment in paediatric patients with refractory epilepsy significantly reduced seizure frequency, with an acceptable safety profile. We defined differences in characteristics and responses to perampanel according to age and concomitant oxcarbazepine or levetiracetam use.
Biró et al. first reported a retrospective study of perampanel use in paediatric patients experiencing refractory epilepsy; they found that the 50% response rate after the first three months of therapy was 31%, with complete seizure control occurring in 8.6% of patients [15]. The results of a multi-centre study indicated a 50% response rate and a 4.8% seizure-free rate over a follow-up of 6.6 months [16]. Two trials with 24 paediatric patients each achieved a response rate of 42% [17, 18]. The first real-world evaluation in Asian paediatric neurology clinics indicated that the rate of 50% seizure reduction was 37.5% and 34.7% at six and 12 months, respectively [19]. A United Kingdom national multicentre observational study on a heterogeneous group of 96 children and adolescents with drug-resistant epilepsy undergoing PER treatment reported an overall responder rate of 18.8% for all seizure types at both six- and 12-month follow-up periods [20]. Yun et al. reported a higher response rate of 68%, and a 23% seizure-free rate after an average of 9.2 months of follow-up in a Korean centre [21]. According to these previous studies, the rates of seizure freedom were 4.8–23% in children and adolescents, whereas the response rates ranged from 30.3% to 68% in children with refractory epilepsy. We demonstrated that 20.8% and 27.4% of paediatric patients achieved seizure freedom, and 46.9% and 51.2% experienced ≥ 50% reduction in seizure frequency by six and 12 months of follow-up, respectively. Our effectiveness and retention rates were high. The use of small increments in perampanel dose and slow titration in the current study likely explain differences in the effectiveness and retention rate outcomes. This is consistent with a previous finding showing that prescription of perampanel in patients with refractory epilepsy using a slow titration schedule facilitated a good retention rate, supporting seizure freedom [22]. The first open-label, prospective study in Korea also suggested that titration speed affects response to treatment, with a slower titration speed resulting in higher response rates relative to those using a faster titration speed. Moreover, the authors showed that slow titration of perampanel could improve the effectiveness and safety profile of perampanel, regardless of concomitant use of other AEDs [23].
A Phase III study of adjunctive perampanel in patients aged ≥12 years established 4 mg/day as the modal dose for treatment of focal seizures [24]. One post hoc analysis also indicated that 4 mg/day was the most common dose for idiopathic generalized epilepsy [25]. The most common dose in our study was also 4 mg/day; thus, our study confirms that perampanel at 4 mg/day confers reduced seizure frequency in paediatric patients. However, there may be little correlation between dose and age. This was demonstrated in Study 232 [5] and 311 [6], showing that perampanel population pharmacokinetic parameters and covariate effects in children and adolescents were similar to those in adults.
With respect to safety and AEs, we did not observe any previously unknown AEs. The most frequent AEs in our study were irritability, somnolence, and dizziness, which were consistent with previous reports [8, 9, 26]. Patients with refractory seizures in our study cohort demonstrated relatively good tolerance, with a 22.9% rate of AEs compared with other series in which AEs were noted in 30.6–67% of patients. All AEs in this study were transient and mild, and a small percentage of patients discontinued the study because of AEs related to perampanel treatment. Aggression was one of the most common AEs leading to discontinuation which is consistent with the open-label extension Study 307 [27]. One possible explanation may be the lower perampanel dosage and slow titration rate used in this study. Slower titration schedules have been associated with fewer AEs overall. Similarly, Ettinger et al. suggested that psychiatric AEs were observed more frequently during the titration phase [28]. Such findings imply that increased caution should be employed during titration of perampanel. The other possible explanation for fewer AEs may be decreased verbal expression in the younger cohort, aged 2-7 years, during titration.
Alongside demonstrating real-world effectiveness and safety, we also attempted to clarify whether age or medication-related factors are associated with superior clinical responses or a reduced risk of AEs. Few studies have addressed this issue. Significant differences were observed concerning the response rates for patients in the two age cohorts. The response to perampanel therapy tended to be higher in patients aged 7–14 years than in the younger cohort aged 2-7 years. This is consistent with previous findings [15, 16]. Rohracher et al. also reported that the likelihood of seizure freedom increased with increasing age [8]. Consistent with previous studies [8-10], younger patients aged 2-7 years had a significantly reduced risk of AEs compared to older children aged 7-14 years in the present study. Kim et al. demonstrated that patient characteristics, including age and sex, did not appear to influence the safety outcomes related to perampanel [25]. The higher rate of AEs among the older patients in this study may be attributed to the slightly faster titration of perampanel and increased verbal expression in older than younger children during titration. Notably, in our study, the mean daily perampanel dose was slightly higher in the cohort with oxcarbazepine than the cohort without oxcarbazepine. Despite the higher overall perampanel dose in the oxcarbazepine cohort, the incidence of AEs was similar across both cohorts with or without oxcarbazepine. This is consistent with other reports [6, 24], which document no differences in risk of AEs between patients receiving, or not receiving, concomitant EIASDs. No significant differences were found between the groups with or without oxcarbazepine in relation to response rate to perampanel, which is consistent with prior observational studies showing that the proportion of responders and seizure-free rates were similar with or without concomitant EIASDs [16]. Similar results were reported in three other studies [29-31]. Moreover, the core Study 311 also suggested that there was no negative impact of concomitant EIASDs on perampanel effectiveness [6]. Patients taking concomitant levetiracetam had greater clinical responses to perampanel than patients without levetiracetam in our study. A previous study showed that the 50% response rate for all partial seizures was higher when levetiracetam was co-administered than when any of the other three AEDs were used with perampanel [32]. A study of perampanel with concomitant levetiracetam for patients with drug-resistant epilepsy showed that perampanel was significantly more effective and seizure-free status was significantly more frequent in patients concomitantly using levetiracetam than in those without levetiracetam [33]. These findings are therefore concordant with those of the present study. The mechanism of action of levetiracetam is unique among AEDs; it is an inhibitor of SV2A-mediated neurotransmitter release [12-14], which has led to the development of new compounds binding to the SV2A site. Similarly, in vitro studies have shown that perampanel is a selective AMPA receptor, involving a novel pharmacological mechanism [34]. Thus, pharmacokinetic or pharmacodynamic interactions between perampanel and levetiracetam may account for the better outcome observed in levetiracetam-treated patients, however, further studies are needed to confirm this.
The packaging of perampanel warns of serious psychiatric and behavioural reactions, including aggression, hostility, irritability, anger, and homicidal ideation, as required by the Food and Drug Administration [4]. Levetiracetam demonstrates a distinct pattern of association with mood and behavioural disorders, including irritability, aggression, agitation, anxiety, and hyperactivity disorder compared with other AEDs in paediatric patients with epilepsy [35]. Thus, the risk of AEs, such as irritability and aggression, is likely to be increased in cases of concomitant perampanel and levetiracetam. However, in this study, aggression was present in two patients without concomitant levetiracetam and in one patient with levetiracetam. Thus, AEs tended to be more common in patients who did not have concomitant levetiracetam compared to those with levetiracetam (27.0% vs 18.8%), although this difference was not statistically significant. Consistent with previous studies of perampanel [23, 33, 36], concomitant treatment with levetiracetam did not tend to affect the incidence of AEs. No serious AEs related to suicidality were reported in our study.
Limitations
There were some limitations in our study. Firstly, this was a retrospective observational study that included a relatively small cohort of patients with refractory epilepsy. Secondly, the study lacked randomization and a control group. Thirdly, the duration of observation and dose of perampanel may have limited our evaluation in some patients. However, this study demonstrated the effectiveness and safety of perampanel therapy in Chinese mainland children and adolescents, which had not been reported previously. Thus, the study findings contribute to the existing knowledge on the effectiveness and tolerability of perampanel in cohorts of different age, as well as concomitant use of oxcarbazepine and levetiracetam.
Conclusion
The effectiveness and safety data reported here demonstrate that adjunctive perampanel treatment is efficacious and well-tolerated in paediatric patients (aged 2-14 years) with refractory epilepsy. These findings support the potential of perampanel as a treatment option for children and adolescent patients with drug-resistant epilepsy.
Supplementary material
Summary slides accompanying the manuscript are available at www.epilepticdisorders.com.
Acknowledgements and disclosures
The work was supported by the Natural Science Foundation of Jiangsu Province (General) Project (BK20201175), Six Talent Peaks Project in Jiangsu Province (WSN-028), Jiangsu Provincial Key Research and Development Program (BE2018661) and Clinical Diagnosis and Treatment of Key Diseases in Suzhou (LCZX201810). The authors thank the many families who participated in this research.
None of the authors have any conflicts of interest to declare.