European Journal of Dermatology
MENUThe potential role of serum polyclonal free light chains as markers of immune activation in psoriatic patients Volume 33, issue 1, January-February 2023
Psoriasis (Pso) is a chronic-relapsing inflammatory disease affecting 2-3% of the general population worldwide [1]. Patients suffering from Pso, especially those with a moderate-to-severe form, may present with various comorbidities such as psoriatic arthritis (PsA), inflammatory bowel diseases, metabolic syndrome and a higher cardiovascular risk [2–6]. Therefore, effective long-term control of this pro- inflammatory status is mandatory [4,5]. Over the last decades, the introduction of biotechnological therapies has represented the therapeutic cornerstone for Pso [7,8]. Nevertheless, the incidence of patients with recalcitrant Pso continues to be relatively frequent [9,10]. Moreover, the development of the use of biological agents in some patients has led to the observation of auto-antibodies, in particular, antinuclear antibodies (ANA), which are usually a sign of immune system reactivation. Thus, it is of interest to search for possible markers, to predict immune reactivation at an early stage, in order to improve the therapeutic response and aid clinicians in their investigation [11]. In view of this, the polyclonal free light chains (FLCs) of the immunoglobulins (Igs) may prove to be useful. These include the κ and λ chains that are synthesized daily and released in the blood at varying quantities (500 mg/day) [12]. Moreover, they are physiologically present in traces in all biological fluids, such as synovial fluid, cerebrospinal fluid, saliva and urine [12–13]. Their half-life of 2-6 hours renders them suitable as sensitive markers of the activation and/or dysfunction of the immune system [13]. Since the first investigations by Bence in 1847 [14], they have been widely used in onco-haematology where their monoclonal form represents one of the parameters evaluated for monitoring therapeutic outcomes and prognosis of immune-proliferative disorders [15,16]. However, FLCs may increase in the case of chronic inflammation or autoimmune diseases, in hypersensitivity reactions or in response to viral infections, however, in these cases, the κ/λ ratio is unmodified [12,17–19]. Only preliminary investigations on FLCs have been performed in patients with Pso. In particular, some authors have found elevated FLCs in synovial fluid and serum of patients suffering from PsA [20,21].
The aim of this study was to investigate the potential role of FLCs as markers of immune activation in Pso patients and determine how they are modulated by biological therapy.
Materials and methods
Study population
The overall study population included 45 patients enrolled at Pso units of the University of Naples Federico II and University of Bologna. Inclusion criteria included a diagnosis of mild-to-severe Pso made by a dermatologist and either ongoing treatment with biologics or without any current systemic therapy. A group of Pso patients (n=15) at baseline (W0) and after 16 weeks (W16) of therapy with adalimumab (ADA) was included for a prospective evaluation of FLCs. In addition, 10 healthy volunteers matched by age and sex were enrolled as controls. Peripheral blood samples were taken from all patients and healthy subjects. Dermatologists were asked to fill out a medical form registering current clinical data, including PASI score, pathological anamnesis and remote pharmacological history for each patient. The experimental protocol was performed according to the current version of Helsinki Declaration, and each subject provided written informed consent before the onset of the study.
Quantitative nephelometric assay
Sera were obtained by standard centrifugation, divided into aliquots, and stored frozen until analysis. Samples were thawed only once and immediately assayed. Serum immunoglobulins (Igs), light chains (LCs) and FLC concentrations were quantified by nephelometry using a BN II (Siemens, Healthcare Diagnostics, Marburg, Germany). In detail, total serum IgG, IgA and IgM as well as LC concentrations were quantified by nephelometric test kits (Siemens, Healthcare Diagnostics); serum FLC concentrations were measured using N Latex FLC kappa and N Latex FLC lambda kit for serum FLCs (Siemens, Healthcare Diagnostics), according to the manufacturer’s instructions. Normal ranges were defined as: κ LC: 1.70-3.70 g/L; λ LC: 0.90-2.10 g/L; LC κ/ λ ratio: 1.35-2.65 for serum LCs, and κ FLC: 6.7-22.46 mg/L; λ FLC: 8.3-27.00 mg/L; FLC κ/λ ratio: 1.35-2.65 for serum FLCs.
Detection of antinuclear antibodies and their specificity
ANA were detected by indirect immunofluorescence (IFI). Patient and control sera were incubated on slides with HEp-2 cells substrate (Kallestad HEp-2 Cell Line Substrate, 12 well slides, Bio-Rad Laboratories, Hercules, CA) at a screening dilution of 1:100 to allow antibody binding. Bound IgG antibodies were detected by incubation of the substrate with a fluorescein-labelled anti-human IgG conjugate, whereas unbound antibodies were removed by rinsing using ZENIT UP (Menarini Diagnostics, Italy). Reactions were observed under a fluorescence microscope equipped with appropriate filters using a ZENIT G-SIGHT (Menarini Diagnostics) with positive value of ≥1:160.
Statistical analysis
Data that passed the normality test were analysed using a two-tailed, t-test, otherwise the Mann-Whitney test was used to calculate statistical differences. Correlations were evaluated with the non-parametric Spearman’s rho test. Considering ANA positivity as an outcome variable, we conducted multivariate logistic regression analysis taking into account the following variables: FLC κ within normal range (6.7-22.46 mg/L) or above normal range (>22.46 mg/L), and FLC λ within normal range (8.3-27.00 mg/L) or above normal range (>27.00 mg/L); also, FLC κ >22.46 mg/L in patients with ongoing therapy for: i) λ >27.00 mg/L in patients with ongoing therapy for: i) p
Size calculation
Since there are few published data to accurately estimate the significant pathogenetic increase in serum FLC levels in Pso patients, we assumed that we would obtain results similar to those reported by Redegeld et al. [22]. Moreover, in order to have an adequate number of samples to establish a minimum hypothetic difference between pre and post anti-TNF-α treatment in terms of serum FLC levels with respect to baseline at 10%, 15 samples were required. A drop-out rate of about 10% was taken into account.
Results
Patients’ characteristics
The enrolled patients comprised 21 males (47%) and 24 females (53%), with a mean age of 58.42±14.86 years and mean PASI score of 12.62±11.08, and 20 of them had ongoing treatment with biologics. Characteristics of the study population are outlined in table 1.
Immunoglobulins, light chains and free light chains
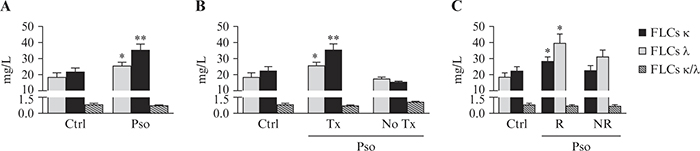
Determinations of total serum IgG, IgA and IgM, as well as κ and λ LCs, from Pso patients and healthy controls are reported in table 2. Although all measurements remained within normal range, the levels of IgG, IgA and both κ and λ LCs were significantly higher in Pso patients than in controls (table 2). In addition, Pso patients showed increased levels of κ and λ FLCs compared to healthy controls (figure 1A). Indeed, the mean concentrations were 25.92±6.03 mg/L and 35.67± 10.90 mg/L in Pso patients versus 18.79±9.17 mg/L (p<0.05) and 22.06±6.56 mg/L (p<0.01) in healthy controls for κ FLCs and for λ FLCs, respectively. To evaluate whether the FLC levels could be influenced by the current therapy, patients were stratified into ongoing treatment with biologics (Pso Tx) and naïve to biologics without any ongoing systemic therapy (Pso No Tx). Interestingly, κ and λ FLCs values were significantly increased in the Pso Tx group but not in the Pso No Tx group (figure 1B).
FLCs in responder and non-responder subjects
To evaluate whether the FLC levels could be influenced by the therapeutic response to ongoing treatment, Pso patients were stratified into responders (R) and non-responders (NR). Responders were defined as patients who had achieved after three months and maintained overtime a PASI response ≥75 to the prescribed biologic agent. Conversely, non-responders were patients who at the time of the enrolment had failed to maintain a PASI response ≥75 in the previous three months. We observed that κ and λ FLC values were significantly increased in the R but not the NR group (figure 1C).
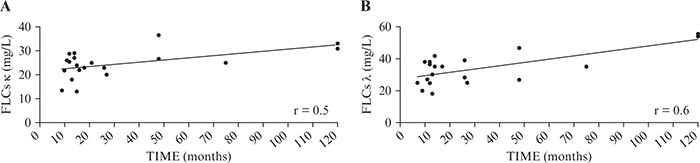
FLCs and treatment duration
Given that biological therapy increased FLCs in our population and in particular in R patients, we stratified R and NR groups according to duration of therapy. R patients were treated with biologics for a significant longer period than NR patients (50.6±41.39 vs 10.9±1.83, mean±SD months; p<0.01). Moreover, both κ and λ FLCs were shown to significantly correlate with duration of therapy (r=0.5 and r=0.6, respectively) (figure 2).
FLCs, ANA and treatment duration
Since it is well known that Pso patients under treatment with anti-TNF-α or other biologics may become positive for antinuclear antibodies (ANA+), the sera from patients with ongoing treatment were assayed also for ANA determination and ANA+ titres were found in 35% of patients (7/20). None of the patients who developed ANA also developed autoimmune disease. Patients with FLC levels above the normal range had higher odds of being ANA+ relative to those with FLC values within normal range (κ: OR=1.86 and λ: OR=2.33, respectively) (table 3). In particular, patients with FLC levels above the normal range and under biological treatment for more than 12 months showed higher odds of being ANA+ relative to patients with FLC levels above normal range but under biological treatment for less than 12 months (κ: OR=3.33 and λ: OR=3.67, respectively) (table 3).
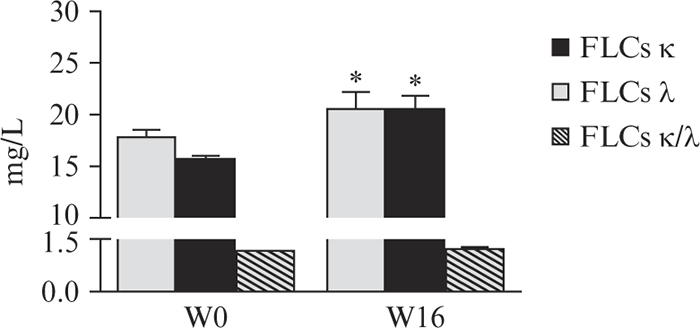
FLCs before starting and during biological treatment
In the light of these results, we also evaluated FLCs in a prospective manner, before starting and during biological treatment. In particular, FLC levels were measured in a group of Pso patients (n=15) before (W0) and after 16 weeks (W16) of ADA therapy. The levels of both κ and λ chains were significantly higher at W16, but remained within normal range (figure 3).
Discussion
In this study, we have shown that both κ and λ FLCs levels significantly increased in Pso patients with respect to controls. Moreover, although all measurements remained within normal range, IgG, IgA and LCs were also shown to be significantly higher. Elevated concentrations of FLCs have been previously observed in several systemic autoimmune diseases such as Sjogren’s syndrome, systemic lupus erythematosus and rheumatoid arthritis (RA) [16,20,23–25]. However, an increase in circulating FLCs has also been recently reported in inflammatory conditions that are not typically associated with autoantibody production [17]. For example, FLCs have been reported to trigger inflammation via activation of mast cells [22]. Passive sensitization of mice with antigen-specific FLCs, followed by antigen challenge, induces an immediate hypersensitivity-type response [26]. This inspired other researchers to investigate FLCs in atopic dermatitis (AD). Firstly, Kayserova et al. showed that there were increased levels of both κ and λ FLCs, as well as their ratio, in a cohort of children with severe forms of AD. Interestingly, the differences in the FLC levels between AD subjects and controls reached very high levels of statistical significance even if the absolute FLC levels were only slightly above normal population values [27]. Conversely, Thijs et al. demonstrated that serum κ FLCs levels in adult AD patients were not increased compared to non-atopic controls and that no correlation with disease severity could be observed [28]. However, mast cells are not the only cellular target of FLCs since neutrophils as well as neural cells have also been found to respond to FLCs. Indeed, FLCs bind to neutrophils and prompt the release of CXCL8, which blocks their apoptosis and contributes to severe chronic inflammation [29]. Moreover, expression of FLCs has been associated with basal-like cancers with aggressive phenotypes in a large cohort of breast cancer patients [30]. In our study, FLCs significantly increased in Pso patients who were receiving treatment with biologics (Pso Tx) but not in those naïve to biological therapy without any ongoing systemic treatments (Pso No Tx) (figure 1B). These results are partially in line with those of Gottenberg et al. who showed that serum κ and λ FLC levels in RA patients (mostly on treatment with anti-TNF-α) were significantly higher than those of controls [23]. To assess possible modulation by the therapeutic response to biologic treatment, we stratified patients into R and NR groups and FLCs were found to be significantly increased only in R subjects. To date, there are no data that clearly correlate FLC levels with the most widely used biological agents for the treatment of chronic inflammatory skin diseases. Nevertheless, previous investigations have revealed that serum FLCs correlate with disease activity and with the clinical response to B-cell depletion therapy with rituximab in RA [31]. Kormelink et al. reported that a significant decrease in κ and λ FLC serum concentrations at three and six months after initiation of rituximab treatment compared to baseline was achieved in only good-to-moderate responding patients [31]. To verify whether the duration of biological therapy may account for the higher FLC levels in R Pso patients, we investigated the association between time on treatment and FLCs levels, revealing a positive correlation. Despite their excellent efficacy, biologic agents can potentially be associated with a variety of immunological effects which influence normal immune responsiveness. Important effects related to the use of these agents include immunomodulation, which might result in outcomes such as infection and autoimmunity. Biologic agent-induced autoimmune manifestations range from the isolated presence of a single class of autoantibodies, such as antinuclear antibodies (ANA), to full-blown autoimmune diseases [32]. Pso patients treated with infliximab (with or without arthritis) were reported to have a higher ANA prevalence [33]. Moreover, Silvy et al. found that 11/20 negative sera of PsA patients before anti-TNF treatment became ANA+ after treatment [34]. In our study, 35% (7/20) of patients with current treatment were ANA+. In particular, patients with κ and λ FLCs above normal range had a higher odds of being ANA+ with respect to those with FLCs values within normal range. Moreover, patients with FLC levels above normal range on treatment for more than 12 months showed an even higher odds of producing ANA. In line with these results, the levels of both κ and λ chains were significantly higher in Pso patients after 16 weeks of ADA therapy, but remained within normal range (figure 3). Thus, an increased FLC level would seem to act as a marker of immune reactivation in Pso patients on treatment with biologic agents, and in particular, an increase might predict ANA production in these patients. The prospective investigation of FLCs, before starting and during biological treatment, also seems to confirm that their increase could be a warning of immune reactivation in relation to the duration of therapy. However, 16 weeks probably represents an insufficient period of therapy to appreciate a very significant enhancement in FLC production. The induction of autoantibodies in patients on treatment with biologics has been suggested to result from a reduction in the concentrations of cytokines that participate in the clearance of nuclear material during apoptosis, thus leading to a greater predisposition to formation of ANAs as well as development of autoimmunity. Moreover, a possible association between cellular apoptotic abnormalities and biological therapy has also been reported in predisposed individuals with consequent release of antigens in the nucleosome and formation of anti-dsDNA antibodies [35]. Because of their rapid turnover, in contrast to total Igs, increased FLC levels could be used to assess this autoantibody production in real-time [36]. In addition, FLC-associated autoantibodies form immune complexes (e.g. with apoptotic cells) which are known to activate the complement pathway and to increase monocyte IFN type-I production that promotes, in turn, plasmablast differentiation and immunoglobulin production [37]. In conclusion, we suggest that the determination of FLC levels has clinical relevance, with a cost/benefit ratio justifying this approach in the clinical management of Pso. Nevertheless, our study is descriptive and was performed using a relatively small sample size of patients, thus further investigations will be necessary to validate FLCs as a valuable tool to predict immunological fluctuations, which occur in these patients during biological therapy, and to monitor their clinical management.
Acknowledgements:
the patients in this manuscript have given written informed consent to publication of their case details.
![]() This work is licensed under a
Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License
This work is licensed under a
Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License