Hépato-Gastro & Oncologie Digestive
MENUAnti-integrins in Crohn's disease: What is their place? Volume 28, supplement 5, November 2021
Figures
- Key words: Crohn's disease, vedolizumab
- DOI : 10.1684/hpg.2021.2228
- Page(s) : 19-23
- Published in: 2021
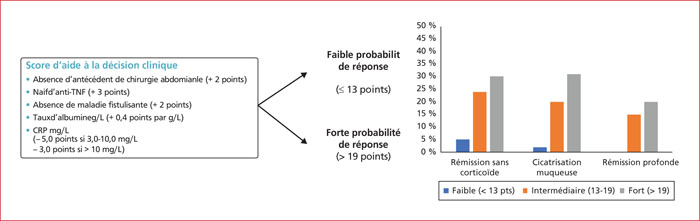
The early assessment of induction has been criticised due to the slower onset of action of vedolizumab, with the plateau of efficacy being around week 14 in the therapeutic trials. Endoscopic response was observed at week 26 in 24.8% of patients and 14.9% had mucosal healing. As with all available treatments for Crohn's disease, prior exposure to anti-TNF drugs greatly reduces the response rates of subsequent biologics. Based on the GEMINI trials, a predictive score for response to vedolizumab was constructed and validated on 5 clinico-biological criteria: absence of previous bowel resection, absence of previous exposure to an anti-TNF, absence of fistulising disease, albumin level, C-reactive protein level The digestive specificity of vedolizumab without systemic immunosuppressive effects suggests theoretically excellent tolerability. The contraindications of this class of anti TNFs are the indications for the other biotherapies, vedolizumab and ustekinumab. There are no head-to-head trials comparing ustekinumab with vedolizumab. In this situation the use of the vedolizumab response prediction score may be a criterion of choice, with a low or intermediate probability preferring the use of ustekinumab. In case of failure of an anti-TNF, a change of class should be considered to vedolizumab or ustekinumab. As with the first line, no comparative studies are available. Three retrospective studies have compared the two molecules, all three being in favour of ustekinumab. All experts agree that vedolizumab should be used in case of failure of ustekinumab.