Hématologie
MENUAntibody-drug conjugate in multiple myeloma Volume 27, supplement 3, Juin 2021
- Key words: Conjugated monoclonal antibodies, immunotherapy, multiple myeloma, belantamab mafodotin, toxic keratopathy
- DOI : 10.1684/hma.2021.1636
- Page(s) : 26-34
- Published in: 2021
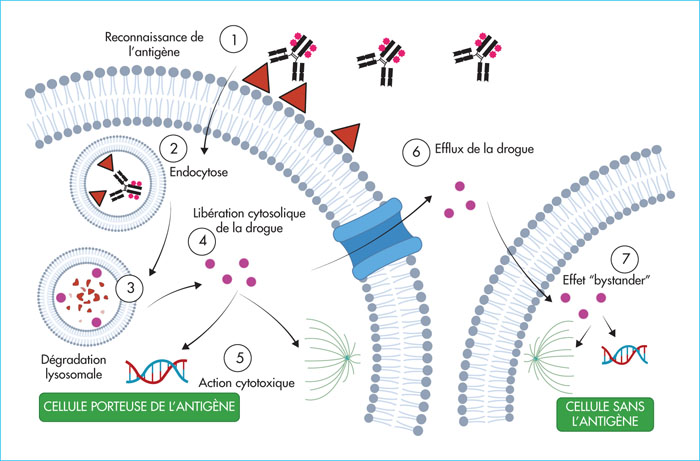
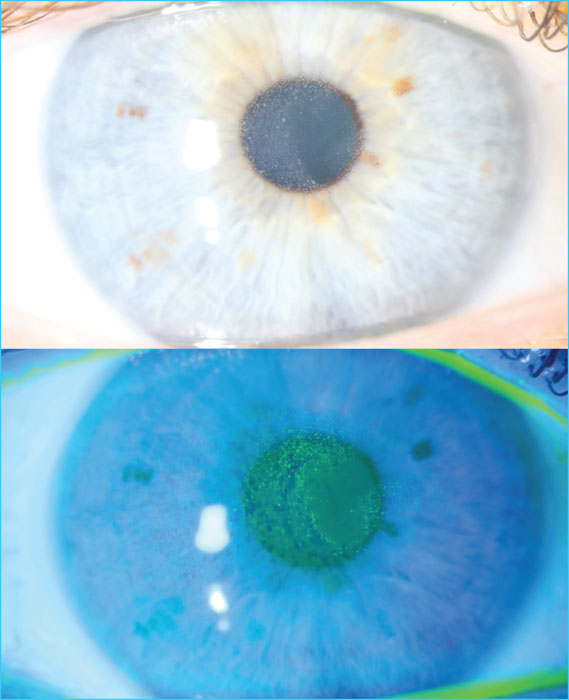
Immunotherapies targeting B-cell maturation antigen (BCMA) have developed rapidly and significantly in multiple myeloma (MM) in recent years. They include chimeric antigen receptor T cells (CAR-T), bispecific antibodies and monoclonal antibody conjugates (ADC). In this article, we discuss the main clinical and biological data available on ADCs. The first in class, belantamab mafodotin, is a monoclonal antibody targeting BCMA, conjugated to monomethyl auristatin F. The DREAMM2 trial led to its approval by the Food and Drugs Administration and the European Medicines Agency, making it the first BCMA-targeted immunotherapy available for the treatment of MM. This drug is currently available in France under a post-approval temporary use programme. It is administered intravenously every 3 weeks, and its use requires precise knowledge of its side effects, particularly ophthalmological, in order to optimise patient management.