Epileptic Disorders
MENUIctal asystole with intercurrent cardiopathy: a complex combination leading to delayed diagnosis Volume 21, issue 6, December 2019
Ictal asystole (IA) is a rare epileptic phenomenon, thought to occur in 0.25-0.4% of seizures (Bestawros et al., 2015). Practically, IA is defined as the absence of ventricular complexes at the EKG lead for more than 3-4 seconds during a seizure (Moseley et al., 2011; van der Lende et al., 2016). Ictal asystole is a continuum of increasing bradycardia during epileptic seizures and can result in cerebral hypoperfusion, thus leading to syncope (van der Lende et al., 2016). Rarely, IA can be prolonged, lasting up to 96 seconds in reported cases with subsequent systemic anoxic consequences (van der Lende et al., 2016). This complication increases the risk of sudden unexpected death in epileptic patients (SUDEP) (van der Lende et al., 2016). The mechanisms of this fatal evolution remains elusive, although it is reasonable to suggest that a depression of respiratory centres (due to deep hypoxia) or fatal cardiac arrhythmias may be involved. Unfortunately, correct diagnosis can be delayed.
We report a case of refractory right temporal epilepsy with ictal bradycardia/asystole. Several routine EEGs and cardiac assessments were conducted. However, given that the patient had significant cardiac comorbidities, investigations were disproportionately focused on eliminating cardiac aetiologies to explain the syncopal episodes. IA was eventually diagnosed, almost 10 years later, with a prolonged video-EEG study.
Case study
Past medical history
A 74-year-old man, born left-handed but ambidextrous by forced use of the right hand, was admitted to our video-EEG monitoring unit in 2017 for diagnosis of recurrent unexplained syncope episodes and presurgical evaluation for drug-resistant epilepsy. Past medical history was significant for a fall from the second floor followed by a few minutes of confusion when he was four years old. No head trauma was diagnosed and the patient did not undergo medical investigation for this incident. He also suffered from mitral insufficiency, initially treated by mitral valve repair in 1995 (and then replacement in 2012). Later, he underwent a triple coronary artery bypass graft surgery in 2011.
Evolution and semiology of the paroxysmal episodes
In 2007, the patient presented with a first episode manifesting with muffled sounds, followed by loss of consciousness, lasting less than a minute. Subsequent episodes were characterized by epigastric auras, followed by impaired awareness, both lasting approximately 30-45 seconds. The epileptic nature of these episodes was confirmed with the capture of an electrographic seizure over right temporo-frontal regions during a routine EEG that same year. Two subsequent EEGs identified right temporal interictal epileptiform discharges as well. Hence, treatment with carbamazepine was initiated, and no further events with epigastric auras were reported for the next seven years. However, he reported syncopal episodes occurring approximately once a year which were attributed to a cardiac aetiology, thus leading to multiple cardiac investigations (see below). These events consisted of a brief loss of consciousness lasting approximately five to ten seconds without aura. Meanwhile, his typical seizures recurred (monthly from 2014), this time characterized by an epigastric sensation, olfactory hallucinations, and impaired awareness. Furthermore, a third type of spell was reported, manifesting with a brief loss of consciousness (now more than ten seconds), without preceding aura, and associated with profuse sweating and urinary incontinence. This type of episode occurred three to four times per year and was also thought to be cardiogenic. A trial of levetiracetam and oxcarbazepine was attempted in order to control the seizures, however, without success.
The patient therefore presented three types of episodes, only one of which was considered epileptic. The main difference considered between the seizures and the other episodes was the presence of an aura (epigastric, olfactory and auditory) during seizures and a motionless state lasting between 10 and 30 seconds during cardiogenic episodes.
Two months prior to admission to our unit, a routine EEG recorded an episode of asystole lasting for 15 seconds. The preceding EEG changes were deemed unclear and suggestive of a probable syncopal event. Brain MRI was normal.
Cardiac assessment
Numerous investigations were performed to determine the aetiology of the syncopal episodes. In 2009, a stress electrocardiogram was normal. Previously, a first 24-hour Holter-ECG was performed in 2008 and revealed a normal background rhythm of 66 bpm, as well as two episodes of remote premature ventricular contractions with 51 ventricular extrasystoles. A second Holter-ECG in 2012 (duration: 17h29’) revealed sinus bradycardia, without any periods of asystole and a third Holter test showed similar findings in 2017. In 2015, a transthoracic cardiac ultrasound documented normal biventricular dynamic, asynchronous septal movement, with bilateral atrial dilatation (severe for the left atrium and moderate for the right atrium). A myocardial perfusion with dipyridamole examination (MIBI) was normal in 2015. The last Holter-ECG in 2017 was similar to the previous (of 2012) and showed a mild bradycardia.
Video-EEG monitoring
When admitted to our epilepsy monitoring unit in 2017, the evaluation revealed right anterior temporal interictal epileptiform discharges, maximum at the F8-T4 electrodes (supplementary figure 1). Eleven electroclinical seizures were recorded over a period of 24 hours at the seventh day of monitoring; all were associated with bradycardia (10 seizures, heart rate range of 35-55 bpm) or asystole (one seizure) (supplementary figure 2). Clinically, the patient reported an epigastric aura followed by dizziness and variable impaired awareness. Electrographically, the seizures began with diffuse attenuation of the tracing, followed by an evolving rhythmic activity over the right temporal leads (F8 T4 [+/- F4]), which then slowly propagated to other regions. Deceleration of the heart rhythm (≤ 55 bpm) occurred with an average latency of 7-23 seconds. Deceleration of heart rate to 30-40 bpm was associated with a decrease in amplitude of the rhythmic activity on EEG, periodic 1-1.5-second triphasic-like sharp waves over right temporal leads, and an independent high-amplitude slow delta activity over the left frontal leads (Fp1 F3). At the end of the seizures, the tracing revealed diffuse slowing, predominantly over the right temporal and bilateral frontal regions, lasting for seconds and up to 10 minutes. The patient was amnestic after most seizures (tested after at least three episodes) during which his heart rate decreased to approximately 30-40 bpm.
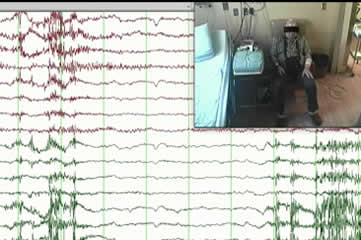
Regarding the episode with IA (the first of 11 recorded seizures), the mean heart rate over the last minute prior to seizure onset was 72 bpm. From the tenth to the eighteenth second of ictal course, a rapid decrease in heart rate was noted (from 70 bpm to 40 bpm). This heartbeat slowing was associated with right temporal-frontal theta-delta rhythmic activity. The heart rate then decreased to 28 bpm after 36 seconds of seizure activity prior to a period of asystole that lasted for 19 seconds. During this period, the EEG transitioned from right-lateralized rhythmic activity and right high-amplitude periodic sharp waves to diffuse slowing followed by EEG flattening from the tenth second after asystole onset (figure 1 and supplementary figure 2). Subsequently, the patient became completely motionless for approximately 19 seconds (similar to during the period of asystole). Approximately nine seconds after asystole termination, brain activity resumed and the patient then manifested a generalized myoclonic jerk while the EEG showed diffuse 2-3-Hz delta activity (see video sequence). The patient was interviewed a few hours later and recalled a “wonderful feeling of leaving his body” at the beginning of the seizure. Our interpretation was that he likely experienced this feeling seconds prior to asystole.
Following the IA episode, the patient had five more seizures within seven hours after which he developed postictal psychosis necessitating treatment with lorazepam and haloperidol. Antiepileptic drug treatment was adjusted and a cardiac pacemaker was implanted as a precautionary measure. No additional syncopal episodes have been reported since (follow-up: 20 months).
Discussion
Ictal asystole is rarely considered as a cause of syncope because the phenomenon is relatively unknown and infrequent. Furthermore, distinguishing ictal impaired awareness from syncopal loss of consciousness may be difficult based on patient recollection and witness testimony. This distinction is especially difficult if the patient does not have a history of epilepsy or if there are consistent cardiac comorbidities that may underlie syncope episodes. Based on a systematic review, Giovannini and Meletti (2014) reported an average delay in diagnosis of 27 months (median: 12 months) in 31 cases in which IA was an early manifestation of epilepsy. We report here a much longer delay in diagnosis of approximately 120 months.
Identification of IA is likely to be easier when there is no intercurrent cardiopathy. Indeed, in patients with an established diagnosis of epilepsy, unexpected collapse and falls occurring late in the course of typical seizures are highly suggestive of IA (Schuele et al., 2007). In practice, however, obtaining the details of the sequence of events leading up to the fall is difficult due to suboptimal patient recollection and frequent lack of reliable witnesses to provide the history. In our case, the clinical picture was blurred by the co-existence of mitral valve disease and multivessel coronopathy which prompted several cardiac investigations instead of continuous video-EEG. In practice, although several routine EEGs had been performed, the diagnosis of IA required recording of a seizure in order to correlate the heart rhythm and loss of consciousness to seizure activity. Considering that routine EEGs usually record 20-30 minutes of brain activity, they are unlikely to capture seizures in a patient with a relatively low frequency of seizures (in our case, once per month). Therefore, routine EEGs failed to link syncope with this patient's seizures, and prolonged video-EEG monitoring was required for accurate diagnosis.
Early diagnosis of ictal bradycardia or asystole could potentially reduce the risk of SUDEP in these patients. For instance, Fava et al. (2015) reported a case of IA in a 56-year-old man whose mother, sister and two brothers also had epilepsy (Fava et al., 2015). In this family, both brothers died suddenly of “indeterminate heart disease” at the ages of 26 and 53, respectively, possibly suggesting that they had seizure-induced asystole as well. IA can be avoided with the insertion of a pacemaker or optimization of anti-epileptic drug therapy which is a modifiable risk factor for SUDEP.
In conclusion, we suggest that video-EEG monitoring should be considered early for patients with epilepsy who exhibit recurrent episodes of loss of consciousness, regardless of whether the episode description is suggestive of cardiogenic syncope rather than seizures. For patients with comorbid cardiac conditions, it is reasonable to perform cardiac investigations first, however, if negative, a low threshold should be established in order to attempt to record the episodes by means of a video-EEG study to rule out IA.
Supplementary data
Summary didactic slides and supplementary figures are available on the www.epilepticdisorders.com website.
Disclosures
None of the authors have any conflict of interest to declare.