Epileptic Disorders
MENUEEG source estimation in a rare patient with cold-induced reflex epilepsy Volume 22, issue 4, August 2020
Temperature-related reflex epilepsy mostly takes the form of hot water epilepsy (Bebek et al., 2001; Zeki et al., 2010; Meghana et al., 2012). This type of epilepsy is often triggered by contact with, or immersion in hot water, usually with a temperature greater than 37 degrees, and manifests with generalized tonic-clonic or complex partial seizures (Szymonowicz and Meloff, 1978; Satishchandra et al., 1988; Kowacs et al., 2005). However, there are few reports and studies on reflex epilepsy associated with low or cold temperatures.
Hot water epilepsy is a rare form of reflex epilepsy that is more common in India and Turkey, with 70% male predominance (Okudan and Ozkara, 2018). The associated interictal EEG shows epileptiform activities or a normal background. The majority of EEG abnormalities are located in the temporal, frontotemporal, temporoparietal, or temporooccipital region on either or both sides (Bebek et al., 2001; Zeki et al., 2010; Meghana et al., 2012). The pathogenesis of hot water epilepsy is hypothesized to be a combination of pathophysiological changes as a response to both physical contact with hot water and the temperature of the water during bathing in genetically and/or anatomically susceptible individuals (Zeki et al., 2010). Although the EEG characteristics of hot water epilepsy are well known, the underlying mechanism and features of brain activity of rare cold-related reflex epilepsy are still unclear.
Case study
The 70-year-old male subject had seizures when he experienced a cold sensation on his body. The seizures started with dizziness, followed by uncontrollable lip and upper limb jittering and shortness of breath. The subject did not experience tongue biting, incontinence, or unconsciousness. Most of the seizures lasted approximately 3-5 seconds, sometimes for tens of seconds. He felt a fever over his body and dizziness after the seizures. The symptoms could be relieved by himself after a certain period of time. The subject had experienced more than a dozen seizures since the onset of the disease, a month and a half ago. The seizures increased to a frequency of several times a day before the subject was hospitalized. Cold temperature was the trigger for the seizures. For example, when the weather became cold, the patient could have seizures when moving from inside the house to the outside. Alternatively, when he laid in bed and lifted the quilt or lifted up his clothes, exposing his abdomen, he had seizures. If the patient did not have a sensation of cold, he did not have a seizure. Head MRI showed multiple lacunar infarctions in the bilateral basal ganglia and a slightly higher signal in the left hippocampus. The family history was negative for epilepsy. The effect of different drug treatments is shown in table 1. The patient still had approximately ten seizures a day after taking levetiracetam or sodium valproate. He had no seizures but occasionally felt dizzy after taking carbamazepine. Oxcarbazepine was taken to maintain long-term treatment. The patient had no seizures during the 10-month follow-up.
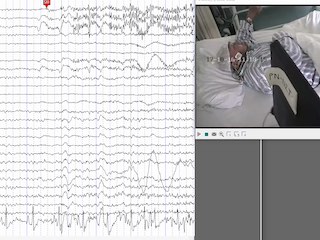
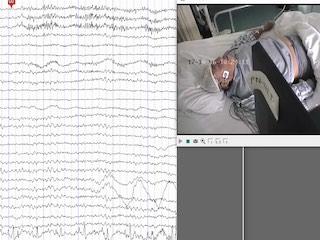
Upon written approval by the Medical Ethics Committee of Luhe Hospital, Capital Medical University, video-EEG recording was performed on the patient before drug treatment. EEG was continuously recorded for approximately four hours (8:32 am-12:35 am) using a video-EEG monitoring system (PN-NET, Beijing Yunshen Technology, China). EEG was recorded using the 10-20 system as well as the left and right sphenoidal electrode sites (SphL and SphR). Three reference electrodes were attached near the Cz position (REF) as the average reference, and on the left and right ears (A1 and A2) as the unipolar reference. The ground electrode (GND) was attached to the forehead. The sampling frequency was 256 Hz. During EEG recording, we performed clinical seizure induction. The patient's jacket was pulled up and his abdomen was exposed to make the patient feel cold. The patient experienced three seizure auras, with no seizures and no abnormal EEG activities. Two seizures were recorded, which lasted for 31 seconds (Seizure 1) and 45 seconds (Seizure 2) (figure 1). His interictal EEG revealed a normal awake background. At seizure onset, β rhythm appeared on the prefrontal electrodes (Fp1, Fpz, and Fp2), ran through the entire ictal period, and disappeared at seizure termination (see video sequence). An approximately 3-second high-amplitude slow rhythm (approximately 1 Hz) appeared at the occipital lobe electrode (O2 in #1 and Oz in #2) in the early stage of the seizures. Then, slow rhythms (approximately 1.5-2.5 Hz) appeared in the mid-late stage of the seizures on the right sphenoid, right temporal lobe, and right frontal electrodes (SPH-R, T4, and F8), which lasted for approximately 10 seconds.
To identify the sources involved in the seizures, we first pre-processed the EEG data. The EEG data were filtered by a 0.5-30-Hz bandpass filter. The power frequency noise was then removed by a notch filter. Average EEG data for each electrode were subtracted to remove the direct component. Large-amplitude artefacts were removed. The primary epileptic sources of the EEG activity were localized by performing source estimation with Brainstorm (Tadel et al., 2011), which is documented and freely available for download online (http://neuroimage.usc.edu/brainstorm). The OpenMEEG method (Gramfort et al., 2010) was selected for forward modelling first. The minimum norm imaging method (Baillet et al., 2001) was then used with the current density map measure for source modelling. We used the non-parametric bootstrap method to test for significant differences between the EEG in the early and mid-late ictal epochs and the EEG in the interictal epoch. Three-second interictal EEG data were chosen as baseline data to estimate the significance threshold. New resampled baseline data were obtained by random sampling with replacement. We then computed the mean absolute values of the resampled data in each EEG channel and calculated the maximum means of all the channels. This process was repeated 1,000 times. The 95% largest of the 1,000 maximum values was considered a significant threshold. For the ictal epochs, the channel was considered significant if the mean of the absolute values of the EEG data was larger than the threshold.
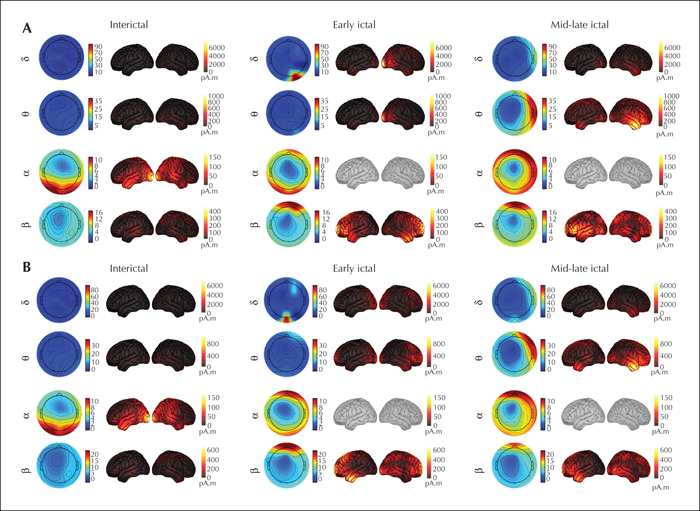
It can be seen from the topographic maps and source maps (figure 2) that the β rhythm appeared in the prefrontal lobe in the two ictal epochs in the two seizures. In the early ictal epoch, a strong δ rhythm mainly appeared in the right lateral occipital lobe in #1 and in the lateral occipital lobe in #2. Then, a strong δ rhythm was found in the right temporal lobe in the mid-late ictal epoch. Similarly, a θ rhythm also appeared in the lateral occipital lobe in the early ictal epoch, especially in #1, and became stronger and shifted to the middle temporal and inferior temporal lobes in the mid-late ictal epoch. A physiological α rhythm emerged in the occipital lobe in the interictal epoch, with no significant change in the ictal epochs.
Discussion
We report a rare patient with reflex epilepsy that was induced by cold sensations, which has never previously been reported in the literature. We describe the drug treatment process and effectiveness of each antiepileptic drug, the implemented clinical seizure induction and EEG recording, and source estimation analysis. For the treatment of cold-induced reflex epilepsy, precautions against precipitating factors are essential, and keeping warm must be the first step for treatment. Daily antiepileptic drug therapy can be considered in the treatment of cold-induced reflex epilepsy. During the drug treatment of this patient, two of the four drugs (carbamazepine and oxcarbazepine) were effective in controlling seizures, especially oxcarbazepine, which can provide a reference for drug treatment in patients with cold-induced reflex seizures in the future. In terms of the ictal EEG discharges, the appearance of β rhythm at the seizure onset indicates that the seizure originated from the prefrontal lobe. For the strong δ and θ rhythms, especially the δ rhythm, that appeared in the occipital lobe in the early ictal stage for a short time and shifted to the right temporal lobe in the mid-late ictal stage for relatively longer time, we suggest that the low-frequency slow rhythms are also related to the cold-induced seizures. These results demonstrate that the prefrontal and temporal lobes are mainly involved in the generation and propagation of epileptic activities, which could provide insight into the neuroelectrophysiological mechanism of cold-induced reflex epilepsy.
This patient is the only patient we have encountered who experienced cold-induced seizures. There were few reports of cold-related reflex seizures in our literature review. Most reflex epilepsy related to temperature takes the form of hot water epilepsy, i.e., the triggering stimulus is immersion in hot water, usually above 37 degrees, and decreased temperature does not cause seizures (Szymonowicz and Meloff, 1978; Satishchandra, 2003; Nechay and Stephenson, 2009). However, a six-year-old girl was reported to have seizures when the temperature of the water was approximately 33 degrees, with right frontotemporal high-voltage slow waves on ictal EEG, and oxcarbazepine caused the disappearance of the seizures (Auvin et al., 2006). Moreover, a 44-year-old man was reported to have seizures when he rinsed his mouth with cold water, with bihemispheric temporal lobe slow waves appearing on interictal EEG with 600 mg of oxcarbazepine taken twice daily (Sala-Padro et al., 2015). These two patients have some features in common with our reported cold-induced epileptic patient regarding EEG abnormalities and drug treatment, i.e., slow waves in the temporal lobe and control of the seizures with oxcarbazepine. From our patient's clinical semiology, although cold temperature could induce seizures, we do not know exactly what part of the body feels cold or how cold leads to seizures. The physiopathology of this cold-induced reflex epilepsy is unclear, but it might also be related to damage in the thermoregulation centre in the subcortical structure of the hypothalamus, as reported in hot water epilepsy (Okudan and Ozkara, 2018).
However, in this study, it should be noted that only one EEG recording was collected, and EEG data were obtained for only two seizures. Also, the EEG data were collected before drug treatment; EEG monitoring and induction tests following each change in antiepileptic drug would allow the effects of each antiepileptic drug on neural activity to be analysed and compared. Lastly, the low spatial resolution of EEG is not very informative, and other neuroimaging techniques such as high-density EEG, MEG, and functional MRI could provide more information.
Acknowledgements and disclosures
This research was supported by a PhD grant from the Faculty of Information Technology at the University of Jyväskylä, Beijing; Science & Technology Commission in Tongzhou District No. KJ2018CX008-15 and No. KJ2019CX014-15.
None of the authors have any conflict of interest to declare.