Epileptic Disorders
MENUDeep brain stimulation of the anterior nucleus of the thalamus in a patient with super-refractory convulsive status epilepticus Volume 21, issue 4, August 2019
Status epilepticus (SE) is a condition resulting from failure of the mechanisms responsible for either seizure termination or initiation which lead to abnormally prolonged seizures (after time point t1) with long-term consequences (after time point t2), including neuronal death, neuronal injury, and alteration of neuronal networks, depending on the type and duration of seizures (Trinka et al., 2015). SE becomes refractory if first- and second-line treatments with anti-epileptic drugs (AEDs) fail to terminate the seizure. SE is defined as super-refractory if SE continues for more than 24 hours after the first administration of general anaesthesia (Shorvon and Ferlisi, 2012).
In serious cases, super-refractory convulsive SE can induce cerebral anoxia, brain dysfunction, and death. ICU patients with refractory SE experience a 41% morbidity rate and a 23% mortality rate. For ICU patients with super-refractory SE, these rates are 55% and 25%, respectively (Kantanen et al., 2017). SE treatments include maintaining the stability of vital signs and internal environment, administration of intravenous AEDs, and anaesthetic drugs with respiratory support for refractory or super-refractory SE. Surgery is important when medicinal interventions fail for refractory SE (Greiner et al., 2012; Bhatia et al., 2013; Bradley et al., 2015; Zeiler et al., 2015), and neuromodulation treatments have been reported in some cases with refractory SE (Valentín et al., 2012; Moseley and Degiorgio, 2014; Zeiler et al., 2015). However, deep brain stimulation of the anterior nucleus of the thalamus (ANT-DBS) as a treatment for super-refractory convulsive SE has seldom been reported.
Case study
Seizure history
A right-handed female patient, born in 1990, suffered from high fever and a generalized tonic-clonic seizure for one to two minutes, and was comatose for three days in 2005. She was diagnosed with viral encephalitis and treated with phenobarbital and mannitol for 14 days, dexamethasone for seven days, and acyclovir and monosialoteterahexosyl ganglioside for 21 days. She subsequently recovered without obvious neurological disabilities. Since then, she has experienced monthly seizures with aura of palpitation and agitation, followed by loss of consciousness, bilateral eyelid spasms, and secondary generalized tonic-clonic seizures, lasting one to two minutes. The patient did not experience incontinence during the seizures, but complained about postictal headaches and tiredness.
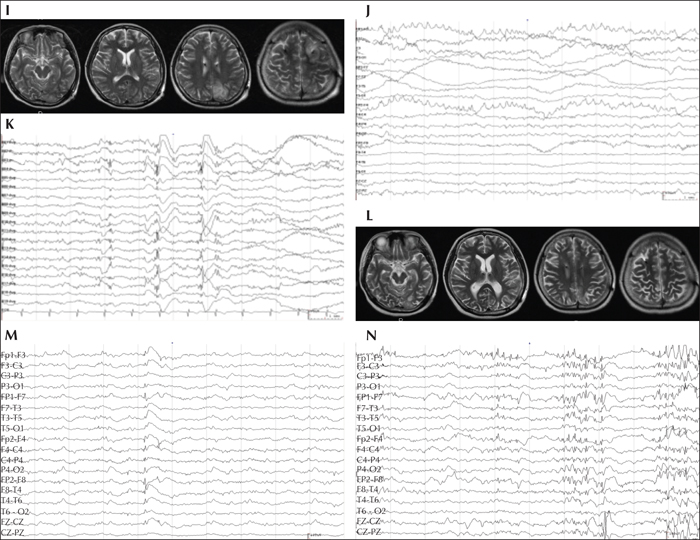
Brain MRI examinations did not reveal any obvious abnormality (figure 1A). The patient became pregnant and was taking both carbamazepine 600 mg/d and valproate 750 mg/d in October of 2014, while still experiencing seizures. On February 7th, 2015, the patient presented with tonic-clonic SE, while four months pregnant. She responded poorly to intravenous injections of methylprednisolone, immunoglobulin, antibiotics, sodium valproate, and diazepam. An intramuscular injection of phenobarbital was also ineffective. General anaesthesia with tracheal intubation and intravenous propofol, midazolam, and cisatracurium besilatea were administrated on the third day after the onset of SE. This continual super-refractory tonic-clonic SE lasted for 12 days. An MRI showed hypoxic-ischemic changes in the right fronto-parietal lobes and bilateral cerebella (figure 1B). The pregnancy was terminated.
The patient suffered two episodes of refractory tonic-clonic SEs for three to five days, and daily generalized tonic-clonic seizures during inter-SE, under the oral administration of valproate at 1,750 mg/d, oxcarbazepine at 1,500 mg/d, and levetiracetam at 2,000 mg/d, and continuous intravenous injections of propofol and diazepam were administrated during tonic-clonic SE. She also presented with severe neurological dysfunction, including inability to answer orienting questions or follow commands, dysphagia, left hemiparesis with level III+ muscle strength of upper limbs and level IV of lower limb, and other functional losses. A super-refractory SE reappeared and lasted for 15 days, starting on March 1st, 2015.
Preoperative evaluation
MRI revealed multiple hypoxic-ischaemic brain lesions in the bilateral cerebella and fronto-parietal lobes (figure 1B). Multiple hypermetabolic foci were also found in the bilateral cerebella and cerebral hemispheres on 18F-fluorodeoxyglucose-positron emission tomography (figure 1D). An interictal scalp EEG revealed generalized low-voltage slow waves with scattered sharp and sharp-slow discharges, and an ictal EEG showed a generalized, very-high-amplitude spike or polyspike wave mixed with fast wave discharges (figure 1E-H). Intelligence quotient, memory quotient, and quality of life could not be tested. The laboratory tests conducted on blood and cerebral spinal fluid did not reveal any related antibody to viral encephalitis or autoimmune encephalitis.
Surgical treatment and outcome
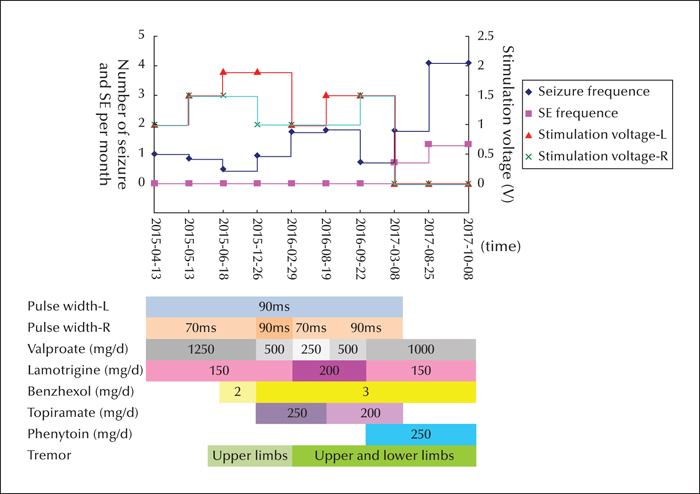
Bilateral ANT-DBS was performed under general anaesthesia and in the prone position on March 27th, 2015 when the patient was 25 years old (figures 1I, L). A stereotaxic head frame was used to insert a quadripolar electrode (1.5 mm in length with an inter-contact distance of 1.5 mm) (PINZ-L302; Pinz Inc., China). After the simulator was activated with 130-Hz stimulation frequency, 1.0 V for bilateral stimulation voltage on April 13th, 2015, the patient experienced monthly seizures but recovered from SE (figure 2). The patient regained consciousness with slow reaction time and was gradually able to walk on her own with left-sided, mild hemiparesis. The epileptiform discharges decreased according to the scalp EEG results (figure 1J).
The patient began presenting with a bilateral upper limb tremor in June of 2015; therefore, valproate was gradually withdrawn and the stimulating voltage decreased. Nevertheless, the tremor became more prominent and, in addition, the seizure frequency and epileptiform discharges increased with a decrease in the stimulating voltage to 1.0 V in March of 2016 (figure 1K). The seizure frequency decreased after the stimulating voltage was increased to 1.5 V in August of 2016. The stimulator was turned off in March of 2017 according to the request of her parents, who insisted that the tremor was a result of the DBS.
After this, the AEDs were adjusted in a local hospital and the patient expressed agitation and a short temper. An ictal scalp EEG showed generalized epileptiform discharges and the focal epileptogenic zone could not be localized (figure 1M-N). From March to August of 2017, the patient suffered four episodes of SE and 14 generalized tonic-clonic seizures, yet the tremor did not improve. The implanted bilateral stimulators were removed under general anaesthesia on August 25th, 2017, but the tremor was not alleviated. The super-refractory SE recurred on October 8th, 2017, and the patient died after 10 days of emergency treatment in the ICU.
Discussion
The patient in this case report suffered repeated events of tonic-clonic SE for two months, with the longest SE lasting for 15 days. Some of her SE was controlled by intravenous injections of propofol, sodium valproate, and diazepam, but the SE recurred after cessation of these drugs. Therefore, the diagnosis of super-refractory SE was confirmed. She had a possible history of viral encephalitis as a 15-year-old (without examination for viral antibodies) and had a history of monthly seizures for 10 years. Nevertheless, neither related antibodies nor autoimmune indications were found in the blood or cerebral spinal fluid after the latest SE, therefore the exact aetiology of the SE remains unclear.
Hemispherectomy is a well-known approach in the treatment regimen of Rasmussen encephalitis-related epilepsia partialis continua. Hemispherectomy is also used as an emergency surgery for other pathological hemispheric changes or laterializable MRI-negative hemisphere epilepsy-related refractory convulsive SE (Moseley and Degiorgio, 2014; Bradley et al., 2015). Epileptogenic and pathological zone removal can stop focal lesion-related refractory SE. Cuello-Oderiz et al. (2015) performed resective surgery (two functional hemispherectomies) in three patients (two of whom were children) with emergency refractory SE or super-refractory SE, and reached favourable seizure control without postoperative death or serious complications. Bick et al. (2016) reported emergency lobectomies in patients with refractory SE related to herpes simplex encephalitis and NMDA-receptor antibody encephalitis, respectively. Bhatia et al. (2013) employed ictal positron emission tomography or single photon emission computer tomography combined with intra-operative cortical EEG to localize the epileptogenic zone in 15 ICU patients with refractory SE. All patients fully recovered from SE after the resective operations. However, resective surgery is not suitable for patients without focal epileptiform discharges on EEG, focal seizures, or positive findings based on neuroimaging. Total-section corpus callosotomy was used in a nine-year-old patient to control the super-refractory SE related to acute viral encephalitis (Greiner et al., 2012).
Neuromodulation surgery is a rational, minimally-invasive therapy for refractory SE patients without a localizable epileptogenic zone. Zamponi et al. (2008) performed vagus nerve stimulation (VNS) in three young children who were six to seven months old and hospitalized in the ICU for refractory SE. All patients reached SE freedom and experienced an improvement in their quality of life during postoperative follow-up. Zeiler et al. (2015) reviewed 28 refractory SE cases treated with VNS and found that 76% recovered from refractory generalized SE. The authors therefore recommend VNS for refractory generalized SE (Grade D evidence). Moseley and Degiorgio (2014) reported a patient with RSE who was successfully treated with external trigeminal nerve stimulation. Centromedian thalamic nuclei DBS was also used to resolve refractory SE in a 27-year-old man ((Valentín et al., 2012) and a 17-year-old boy (Lehtimäki et al., 2017). Furthermore, electroconvulsion therapy (ECT) is reported in patients with refractory SE, and SE cessation was obtained in 80% of cases, and complete recovery was achieved in 27% of patients (Lambrecq et al., 2012; Yang and Wang, 2015).
The SANTE study demonstrated the efficacy of ANT-DBS in controlling seizures and improving patient quality of life based on prospective, controlled research and long-term follow-up (Salanova et al., 2015). Moreover, ANT-DBS is reported to induce anti-apoptotic and anti-inflammatory effects in epileptic rats (Ferreira et al., 2018). Accordingly, ANT-DBS could be useful to control SE and protect the brain from secondary injury in patients with SE. Lee et al. (2017), successfully treated a 17-year-old female patient with refractory SE using ANT-DBS. Furthermore, we have performed DBS in a patient with bilateral temporal lobe epilepsy, hypothalamus hematoma, and intractable epilepsy without a localizable epileptogenic zone. However, an external trigeminal nerve stimulator was not available in China at the time, and centromedian thalamic nuclei DBS and ECT have not been used for intractable epilepsy in our centre. Therefore, we performed ANT-DBS in this patient.
Our patient is the first reported case with super-refractory SE who was SE-free for two years with ANT-DBS and subsequently died as a result of repeated super-refractory SE after the removal of the simulator. Therefore, this case shows the effectiveness of ANT-DBS with both positive and negative outcomes. The patient presented with preoperative interictal and ictal generalized discharges on scalp EEG, and these generalized discharges disappeared when the ANT-DBS stimulating parameters reached 1.9 V on the left side and 1.5 V on the right side, which was accompanied by SE freedom and obvious seizure reduction. However, the generalized discharges and SE reappeared after the power of the stimulator was switched off or after removal of the stimulator. It was inferred that ANT-DBS can prevent the synchronization and diffusion of the epileptic discharge which may relate to the SE control.
The surgeries conducted in previous reports, including resective and neuromodulation surgery, were mostly performed one to three months after the refractory SE diagnosis. Our patient also underwent ANT-DBS two months after her SE diagnosis. She continued ANT-DBS treatment until she presented with multiple brain injuries, hemiplegia, and a delayed limb tremor. Therefore, early surgical intervention should be taken into consideration for patients with super-refractory SE. Nevertheless, all the previous clinical studies on surgical treatment in patients with acute refractory SE are either based on case reports or retrospective case group studies with a small sample size. These findings indicate the urgent need for a prospective, large-sample, controlled study.
Acknowledgements and disclosures
The authors are grateful to the patient's family for their long-term cooperation. We also appreciate the contribution provided by Mrs. Xiaoyu Shang and Dr. Ping Ding in the Capital Epilepsy Therapy Center. This research was supported by Chinese National Nature & Science Foundation (81771388) and Brain Research Fund of Beijing Municipal Science and Technology Commission (Z171100000117014) and Open Fund of Beijing Key Laboratory of Epilepsy, Sanbo Hospital, Capital Medical University (2017DXBL01). These funds did not contribute to the study design, data collection and analysis, interpretation of data, or writing of this report.
None of the authors have any conflict of interest to declare.