Epileptic Disorders
MENUCoregistration of multimodal imaging is associated with favourable two-year seizure outcome after paediatric epilepsy surgery Volume 19, issue 1, March 2017
Presurgical evaluation of intractable epilepsy typically includes both anatomical (i.e. MRI) and functional imaging studies (i.e. PET, SPECT).Each modality has strengths and weaknesses with respect to spatial and temporal resolution, and with respect to functional or anatomical relevance (Olson and Perry, 2013). Delineating an anatomical lesion on MRI congruent with EEG-defined seizure onset is favourable, though functional imaging studies can further define the extent of the epileptogenic zone beyond the MRI-visible lesion, and are especially useful in delineating epileptogenic cortex in MRI-negative cases (Chassoux et al., 2010).As favourable outcome following epilepsy surgery is primarily dependent on complete resection of the seizure onset zone, techniques which improve presurgical localization would be expected to improve outcome (Krsek et al., 2009). Imaging data has traditionally been reviewed in two-dimensional planes using side-by-side or individual visual analysis. Coregistration places multiple imaging modalities into a common anatomical space and can increase the diagnostic value of the data by overcoming intrinsic limitations of individual modalities and improving localization (Olson and Perry, 2013).
While it is generally accepted that coregistered imaging leads to improved postoperative seizure control, there is a paucity of research to support this assertion. Multiple studies have demonstrated, through comparison with intracranial EEG localization or based on favourable postoperative seizure control, that coregistering functional imaging modalities to MRI improves localization of the seizure onset zone. For example, coregistration with SPECT and MR spectroscopy in adult temporal lobe epilepsy results in increased sensitivity of focus lateralization to 100% (Doelken et al., 2007). Subtraction SPECT coregistered to MRI (SISCOM) demonstrates particular value in non-lesional temporal lobe epilepsy (Brinkmann et al., 2000; Wellmer et al., 2002; de Ribaupierre et al., 2012; Sulc et al., 2014). Fluoro-deoxyglucose-PET/ MRI coregistration aids in localization, identifying 95% of MRI-negative focal cortical dysplasias with favourable postoperative seizure-free rates (Chassoux et al., 2010). While these studies support the localizing value provided by coregistration, they do not clarify whether the implementation of coregistration impacts seizure outcome following surgery compared to traditional methods of individual or side-by-side visual analysis.A recent study by Rubinger et al. (2016) demonstrated improved seizure control after paediatric epilepsy surgery following a change in imaging protocol which included utilization of 3T MRI, as well as increased use of magnetoencephalography (MEG) and PET. They did not investigate the impact coregistration alone had on their outcomes, though it was a part of their new imaging protocol. There is no level 1 evidence to support that coregistration techniques can impact postoperative seizure outcome, and design of such studies has been limited by sample size, lack of control populations, lack of randomization, and non-blinded assessment (Gaillard et al., 2011).
Understanding the value of coregistered imaging is prudent in paediatric epilepsy where extratemporal and MRI-negative epilepsy is common. Because post-processing techniques require time and resources to complete, it is essential to understand the value to the patient to justify the expenditure. Our centre used individual and side-by-side analysis of images prior to 2009 and added coregistration of neuroimaging subsequently. We hypothesized that a change in our imaging protocol to include review of coregistered imaging would be associated with improved postoperative seizure control in paediatric patients undergoing epilepsy surgery.
Methods
We reviewed medical records of patients with intractable epilepsy followed in the Cook Children's Comprehensive Epilepsy Program who underwent pre-surgical evaluation and resective epilepsy surgery between January, 2006 and December, 2012. Patients were included if they were less than 21 years of age, had at least one year of post-operative follow-up data, and underwent at least one anatomical and one functional neuroimaging study as part of their pre-operative evaluation. For patients who underwent a repeat epilepsy surgery, only the initial evaluation and surgery were evaluated. Patients undergoing preoperatively-designated palliative procedures, intended to result in seizure reduction but not seizure freedom were excluded. We separated patient evaluations into those with exclusively individual modality or side-by-side review (pre-8/2009, pre-MMI) and those with coregistered imaging (post 1/2010, MMI).Following presurgical evaluation, patient data was reviewed and surgical plans made at an epilepsy surgery conference attended by neurologists, epileptologists, neurosurgeons, neuropsychologists, and neuroradiologists. The study protocol was reviewed and approved by the IRB.
Abstracted data included baseline demographics, as detailed in table 1 table 1. All patients had VEEG as part of their presurgical evaluation and all neurophysiology data was reviewed by board-certified neurophysiologists. All had brain MRI performed with a minimum of 1.5 T magnet strength and at least one thin-slice (1-mm) T1- axial or coronal image in addition to T2- weighted axial images, and axial and coronal fluid-attenuated inversion recovery sequences. MRI findings were described as lesional (i.e. gyral abnormalities, focal signal changes, hippocampal sclerosis) or non-lesional (i.e. diffuse atrophy or normal). Other presurgical imaging was completed at the discretion of the primary epileptologist.PET was a routine component of pre-operative evaluation throughout the period under study and was completed in nearly all patients. When PET was not performed, it was typically secondary to lack of insurance approval. SPECT studies were used in patients with multiple lesions on MRI, MRI-negative patients, or those with poorly localized seizure onset based on EEG. All SPECT data included ictal and interictal data with subtraction imaging. SPECT protocols remained stable other than a brief period in 2010 when isotope was changed due to national shortages. PET data was viewed with coregistered CT in the pre-MMI group. MEG was available at our facility at the beginning of 2010 and was utilized in only four patients in this study. Neuroimaging was reviewed by one of two neuroradiologists. Functional imaging results were characterized as congruent, incongruent, or normal based on the impression following epilepsy conference data review and confirmed by subsequent review of radiology reports and images. Congruent studies demonstrated a dominant region of interest within the same cerebral lobe as seizure localization based on ictal VEEG data. For normal and incongruent studies, a dominant region of interest was not demonstrated or the region was not within the same lobar localization as that based on EEG data.
Operative data included whether surgery was temporal or extratemporal. Procedures involving the temporal lobe, but extending beyond, were considered extratemporal. Surgeries were described as unilobar or multilobar, by operated hemisphere, and by surgery type (recorded as lesionectomy, lobectomy, hemispherectomy). The surgical plan was defined as either a single stage or a two-stage if invasive electroencephalography was required. The number of electrodes implanted was determined from surgical reports. Finally, postsurgical data included pathology categorized as congenital (i.e. cortical dysplasia, hamartoma, developmental tumours) or postnatal (i.e. infectious, hypoxic, vascular), and seizure frequency outcome using Engel's classification (Engel et al., 1993). Seizure frequency was abstracted from clinic notes based on parental report at each visit, and routinely recorded as the average number of seizures per unit time (i.e. day/month/year).Outcome was reported as “Favourable” (i.e. Engel Class I) or “Unfavourable” (i.e. Engel Class II-IV).
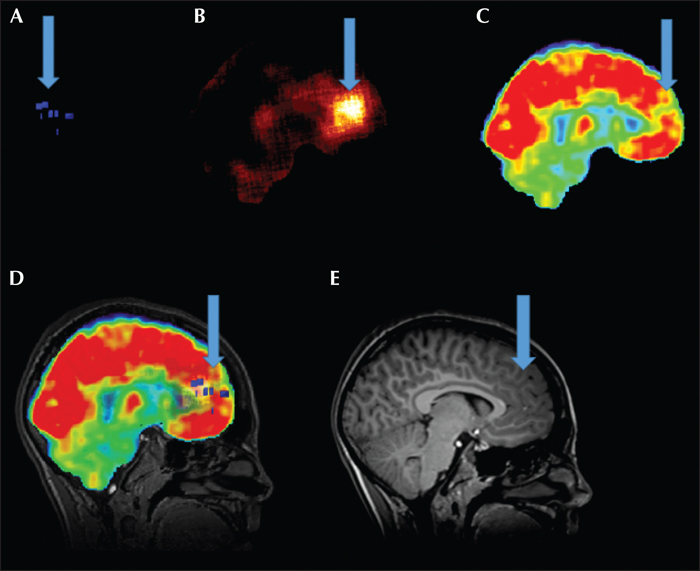
In the MMI group, functional and anatomical imaging datasets were squared to a 256 by 256 matrix and then coregistered to T1-weighted thin-slice MRI using automated methods (Multimodality Brain) in Hermes Version Gold 2.10 (Hermes Medical Solutions). Automated methods have previously been described elsewhere (Studholme et al., 1997). Following visual inspection of registration for accuracy, coregistered datasets were imported to Amide (Version 0.9.2, http://amide.sourceforge.net) to allow multimodal overlay of imaging data and adjustments of transparency and thresholds (Loening and Gambhir, 2003). A skull-stripped cerebral volume mask was created from the coregistered MRI utilizing BrainSuite and then applied to SPECT and PET data to remove extra cerebral tissue (Shattuck and Leahy, 2002). Ictal and interictal SPECT studies were normalized and then subtracted within AMIDE, leaving hyperperfusion blobs for review. Images are viewed as single studies (figure 1 A-C, E) and together as overlays (figure 1D).
Baseline patient characteristics and preoperative evaluation were compared between groups using Pearson χ2 for discrete variables and t-tests and ANOVAs for continuous variables.To investigate whether the number of imaging studies per patient had an impact on outcome, a binary logistic regression model was employed.The response variable category favourable surgical outcome was treated as the reference category. Predictor variables were visit (12 and 24 months) and group (pre-MMI and MMI). As our evaluation protocol had changes beyond implementation of coregistration (i.e. use of 3T MRI), we sought to account for the cumulative impact confounding variables had on outcome between the two groups. Stabilized inverse probability-weighted estimators (i.e. propensity scores) were specified to adjust for the effect of each confounding variable. Propensity scores were estimated based on age at onset, seizure duration, AED exposure, number of seizure types, EEG results (focal versus multifocal), seizure frequency, MRI magnet (1.5T vs 3T), MRI lesional status, type and number of imaging studies, stage of surgery (one vs two-stage), and type of resection. The propensity score-adjusted variables, along with year of surgery as a continuous variable, were then evaluated in a binary logistic regression with treatment group predicting outcome at one and two years to determine the relationship of coregistration on seizure control. To evaluate the proportion of two-stage surgeries and between group differences in the number of electrodes used to characterize the epileptogenic zone, we utilized a general linear model controlling for potential covariates. The independent variables evaluated included patient demographics, imaging results, surgery type (one versus two-stage), resection type, operated hemisphere, and pathology. Data were analysed using the SPSS 19 statistical package with the level of significance defined as p<0.05.
Results
Population characteristics
Two hundred and four patients underwent resective surgery during the inclusion period. We excluded 21 palliative cases (10 incomplete resections with bilateral seizure onset and 11 callosotomy [pre-MMI 4]), 12 due to lack of follow-up data [pre-MMI 8], 18 with prior resections [pre-MMI 11], 30 without both an anatomical and functional study [pre-MMI 16], and eight over 21 years of age at the time of evaluation [pre-MMI 4].
Thus, 115 patients were included in the final evaluation. Sixty-eight (59%) had coregistration of imaging. Patient characteristics are presented in table 1. The MMI group had a higher proportion of monthly or greater than monthly seizures (χ2[1]=8.35; p=0.004), while the pre-MMI group had proportionately more patients with daily seizures (χ2[1]=6.87; p=0.009). Patients in the MMI group were exposed to fewer AED trials prior to evaluation for epilepsy surgery (p=0.018), but duration of epilepsy prior to surgery did not differ.
VEEG and imaging variables are presented in table 2 table 2. VEEG captured seizures in all patients. The proportion of patients with unifocal and multifocal EEG onset were similar between groups. MRI was normal in 16% of patients in the MMI group and 32% in the pre-MMI group (χ2[1]=3.93; p=0.047). Patients in the MMI group were evaluated with more imaging studies (χ2[2]=25.47; p<0.001), though outcome was not changed based on use of additional studies alone (χ2[2]=2.79; p=0.247). We found no significant difference in one vs. two-stage surgical plan or outcome based on which type of functional study was completed as part of the pre-surgical workup.
Operative data
Surgical descriptors are presented in table 2. Surgical procedures performed following coregistration revealed a significant decrease in the number of two-stage surgeries (66% vs 47%; χ2[1]=4.01; p=0.045). The mean number of electrodes used in two-stage surgeries remained consistent (62 vs 54). Two-stage surgeries were more often performed in patients with non-lesional MRI (77% vs 48%; χ2[2]=6.65; p=0.010), incongruent MRI and EEG (72% vs 42%; χ2[2]=10.56; p=0.001), or extratemporal onset (73% vs 35%; χ2[1]=17.43; p<0.001). The majority of patients in both groups underwent temporal resection. Coregistration was associated with improved outcome in extratemporal lobe procedures (59% vs 25%; χ2[1]=12.18; p<0.001) and temporal lobe procedures (69% vs 38%; χ2[1]=8.67; p=0.003).
Seizure reduction
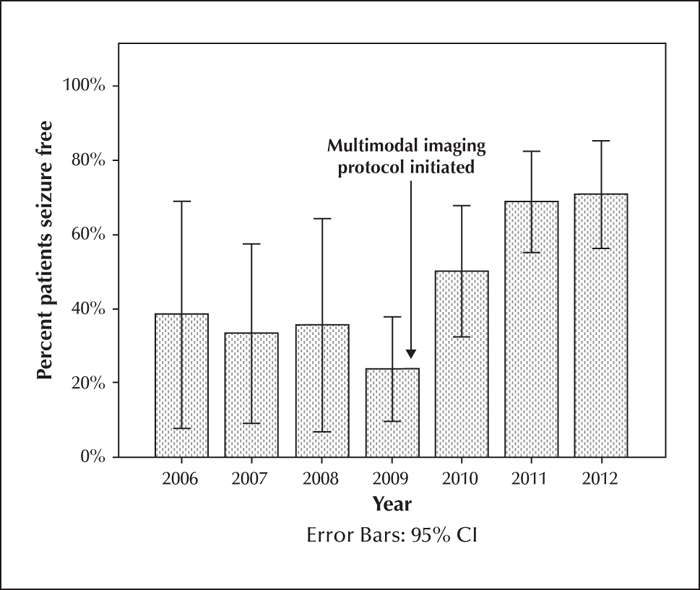
Patients evaluated with coregistration were significantly more likely to be seizure-free at one and two years. At 12 months, the use of coregistered imaging significantly predicted favourable seizure outcomes (Engel Class I) (χ2[2]=8.64; p=0.034). The odds ratio for favourable seizure outcome at 12 months following coregistered imaging was 5.42 (95% CI [1.04-28.32]; p=0.045). At two years, the use of coregistered imaging significantly predicted favourable seizure outcomes (Engel Class I) (χ2[2]=22.08; p<0.001). The odds ratio for favourable seizure outcome at two years following coregistered imaging was 21.64 (95% CI [2.94-159.51]; p=0.003). There was a dramatic improvement in favourable outcome after the use of coregistration in 2009, which remained stable in subsequent years (figure 2).
Discussion
The implementation of coregistered neuroimaging as a part of presurgical evaluation for intractable epilepsy was associated with postoperative seizure freedom at one and two years and fewer invasive subdural monitoring evaluations. While coregistered imaging has been demonstrated to influence surgical planning over side-by-side visual analysis, the impact on seizure outcome has not been as well described (Doelken et al., 2007; Salamon et al., 2008; Seo et al., 2011; Zhang et al., 2013; Chandra et al., 2014; Fernandez et al., 2015; Nowell et al., 2015). Nowell et al. (2015) found coregistered imaging led to a change in surgical strategy in 34% of patients and to changes in electrode placement in 81% of patients. This particular study focused on the process of surgical planning and electrode placement, utilizing 3-dimensional brain and vascular reconstruction, but did not investigate whether coregistration had an impact on seizure-free outcome. Salamon et al. (2008) investigated the utility of PET/MRI coregistration by comparing cohorts before and after the addition of this technique to their presurgical protocol. They found additional benefit for localization of epileptogenic foci, especially in MRI-negative patients, resulting in less two-stage surgeries. They did not compare seizure reduction before and after coregistration was implemented. Seo et al. (2011) noted multimodal imaging changed surgical plans, resulting in smaller craniotomy size and the need for fewer electrodes during invasive monitoring, but did not report how this impacted patients’ seizure-free outcome. Finally, Rubinger et al. (2016) demonstrated that a change in presurgical imaging protocol, utilizing 3T MRI, MEG and coregistered PET, improved outcome, but did not separate the value of coregistration from their change in imaging modalities, and only included one year of postoperative follow-up. In the present study, we demonstrate a positive impact on postoperative seizure freedom after implementing coregistered imaging into our standard presurgical protocol. We found a reduction in two-stage epilepsy surgeries and sustained seizure freedom at two years post-surgery, an important finding given outcome can decline with longer follow-up duration but remains fairly stable two years post-surgery and beyond (Krsek et al., 2009; Liang et al., 2012). The value of coregistered analysis is potentially more impactful in childhood, as paediatric epilepsy is often secondary to subtle malformations of cortical development which may go undetected by MRI alone. It is likely that the use of coregistered imaging improved our confidence with regards to the region of seizure onset, thus we felt invasive monitoring for localization was required for fewer cases. It is also likely that improved localization led to more complete resections of the epileptogenic zone, leading to improved seizure-free outcomes in our cohort.
The cohort included a wide range of ages, with 22% MRI-negative and 52% extratemporal.Pre- and post-MMI cohorts were similar for baseline epilepsy characteristics. The MMI group more often had monthly or greater seizure frequency, representing a lowered threshold for considering patients for epilepsy surgery at our institution over time. While a higher seizure frequency would be a predictor of poor outcome, we controlled for this variable in our analysis. The decrease in number of AED trials before surgery in the MMI group also reflects a move towards recommending epilepsy surgery after two failed AEDs. The duration of epilepsy prior to surgery, a variable shown to impact outcome, was not significantly different despite the lower threshold for recommending surgical therapy (Englot et al., 2013; Mequins et al., 2015).
We recognize that ascribing improvement in outcome solely to the use of coregistration is difficult, as inevitably there will be improvements in imaging quality, centre expertise, and differences in patient populations over time. Likewise, this study uses known datasets reviewed retrospectively with some of our methods of image analysis lacking validation, leading us to conclude coregistration is associated with favourable seizure outcome, though not the sole cause of it. However, given the absence of any randomized, blinded, and controlled data to date and the low likelihood of such studies being carried out in the future, we felt it important to control for as many of these confounding variables as possible in an effort to demonstrate the value of multimodal coregistration. There were fewer MRI-negative cases identified in the MMI group, which may be due to better recognition of subtle lesions using the 3T magnet or better recognition of subtle lesions when functional imaging is coregistered, as has been previously demonstrated (Chassoux et al., 2010; Rubinger et al., 2016). Forty-seven percent of patients in the pre-MMI group had 3T MRI and there was not a significant difference in the number of lesional patients within the group based on magnet strength. Thus, it is more likely that coregistration of other imaging to MRI improved recognition of lesions, resulting in fewer MRI-negative cases in the MMI group (Brinkmann et al., 2000; Wellmer et al., 2002; Chassoux et al., 2010; de Ribaupierre et al., 2012; Sulc et al., 2014).
MMI patients had more functional studies as part of their evaluation and this was largely due to increased numbers of SPECT scans, as almost all patients had PET scans. MEG was the only functional study not available to all patients during the evaluation period, but was performed in only a small number of MMI patients (n=4). Regardless, outcome was not influenced by the type or number of functional studies performed.
Patients evaluated with coregistered imaging underwent fewer invasive EEG monitoring procedures which underscores the value of improved epileptogenic zone localization through presurgical evaluation. This reduction in invasive monitoring was most likely secondary to improved confidence in localization following the presurgical workup when multimodal image coregistration was employed. Two-stage surgeries were more often performed in patients with incongruent imaging and EEG data, extratemporal onset, and non-lesional MRI, which is not unexpected. There was no significant change in the number of electrodes implanted when extraoperative monitoring was performed. Limiting the need for invasive monitoring decreases risks of adverse effects such as infection, haemorrhage, and increased intracranial pressure (Arya et al., 2013). Reduction of two-stage surgeries would be expected to reduce costs of care, by avoiding additional surgical costs and reducing the duration of hospitalization. This finding alone is an important reason to consider utilization of coregistered imaging techniques.
Multimodal coregistered neuroimaging had a marked association with favourable post-operative seizure control. Overall, approximately 80% of our cohort experienced initial favourable outcomes regardless of whether coregistration was employed, similar to other paediatric series (Wyllie et al., 1998; Krsek et al., 2009). We saw a decrease in favourable outcome within the first year, as has been reported in other studies (Liang et al., 2012). This decrease was marked in the pre-MMI group, while those evaluated with coregistered imaging had sustained favourable outcome with 64% remaining Engel Cass I at two years post-operation. This may be attributable to improved localization of the epileptogenic zone resulting in complete resection of the epileptogenic focus, the best predictor of post-surgical outcome (Krsek et al., 2009). To support that improved outcome was related to coregistered imaging and not cumulative contribution of multiple pre-operative factors, we calculated propensity scores of the most common variables known to impact epilepsy surgery outcome. This analysis included variables of significant difference between the groups (i.e. seizure frequency, MRI lesional status) and changes in our evaluation process (i.e. 3T/1.5T magnet, number and type of functional studies). Accounting for these covariates, patients undergoing MMI analysis remained significantly improved at both one and two years post-operation.
This study is strengthened by comparing two surgical cohorts, one evaluated with and one without coregistered imaging. We recognize several limitations of design including the retrospective nature. We cannot account for the impact experience may have had on our outcomes, though year of surgery did not impact outcome. Our surgical programme has been active since 2001, thus the primary participants had several years of experience together before the cohort in this study was evaluated and the surgical procedures were primarily performed by the same surgeon (DD). Favourable surgical outcome at two years remained fairly stable at <50% until 2010 when favourable outcome increased to >50%. This abrupt change also supports the impact of our coregistered imaging more than experience, as the change would have been more gradual and there were no other significant modifications that year to explain such a difference otherwise. While we cannot possibly account for all improvements in imaging technology over the six years included, we found no difference based on the type of imaging study performed, no change in proportion of congruent studies over time, and there was no change in protocol other than viewing data as coregistered sets in 2010 to explain the increase in favourable postsurgical outcomes. We did not consider whether patients remained on AEDs postoperatively. It is the protocol of our facility to continue AEDs until at least two years after surgery, thus this is not likely to impact outcome differences between groups at the time points we investigated. We did not include unintended functional deficits in our dataset, thus we cannot comment on the impact coregistered imaging has on adverse postoperative functional outcome.
The use of multiple imaging modalities in the presurgical evaluation of intractable epilepsy is common. Coregistration of multiple modalities to anatomical imaging is an important component of the non-invasive presurgical evaluation, and is associated with improved seizure outcome when compared to traditional analysis. At the least, implementation of coregistration techniques likely improves confidence in localization and increases recognition of subtle lesions. Coregistered imaging may reduce the need for invasive neurophysiological monitoring and contributes to increased rates of postoperative seizure freedom up to two years after paediatric epilepsy surgery, a favourable predictor of long-term outcome. Coregistration of neuroimaging data should be considered as a component of presurgical evaluation for children with intractable epilepsy.
Supplementary data
Summary didactic slides are available on the www.epilepticdisorders.com website.
Disclosures
None of the authors has any conflict of interest to disclose.
* Portions of this work were submitted and presented at the American Epilepsy Society 68th Annual Meeting, Seattle, WA, December 2014.