Hépato-Gastro & Oncologie Digestive
MENUTyrosine Kinase Inhibitors: Interest of the pharmacology and main errors to avoid Volume 26, issue 2, Février 2019
- Key words: tyrosine kinase inhibitors, pharmacology, dosage
- DOI : 10.1684/hpg.2019.1747
- Page(s) : 178-85
- Published in: 2019
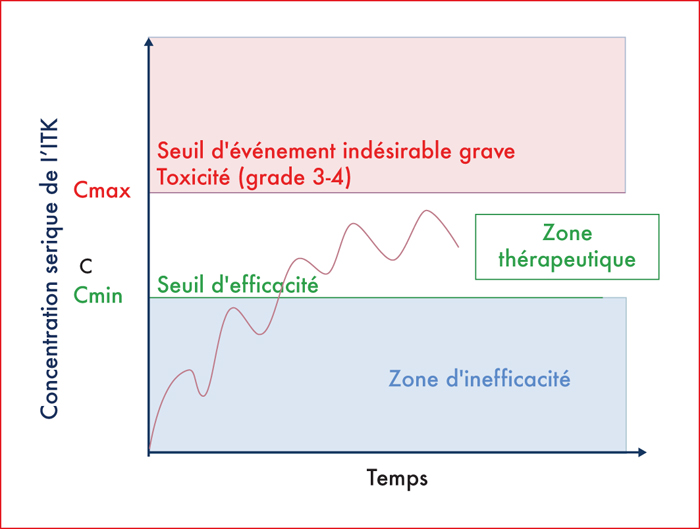
Recent years have been associated with the development of tyrosine kinase inhibitors (TKIs) in digestive oncology. TKIs inhibit the phosphorylation of tyrosine kinases or serine/threonine kinases in the cell membrane. With TKIs, it is not uncommon to observe a dose reduction or even a discontinuation of treatment. All this calls for the development of a personalized dosage in order to optimize treatment. The very high incidence of adverse events suggests that TKIs have a narrow therapeutic window. ITKs have a low bioavailability, lower than 30%, which explains the large variation between individuals. Current validated pharmacological models exist for imatinib, sorafenib and sunitinib to allow a routine pharmacological patient monitoring.Several factors have been identified as modifying the drug concentration or the area under a curve (AUC) of some ITKs, including intake splitting, gastrectomy, bariatric surgery, proton pump inhibitors and are to be considered when prescribing TKIs. Also, some potential errors regarding prescription modalities were identified (time of intake, distance from meals, risk of non-observance, and intake of inducing products).
![]() This work is licensed under a
Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License
This work is licensed under a
Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License