Hépato-Gastro & Oncologie Digestive
MENUSelective and non-selective JAK-STAT in Inflammatory Bowel Disease Volume 27, supplement 4, Novembre 2020
- Key words: Inflammatory Bowel Diseases, JAK-STAT pathway, tofacitinib
- DOI : 10.1684/hpg.2020.2039
- Page(s) : 43-51
- Published in: 2020
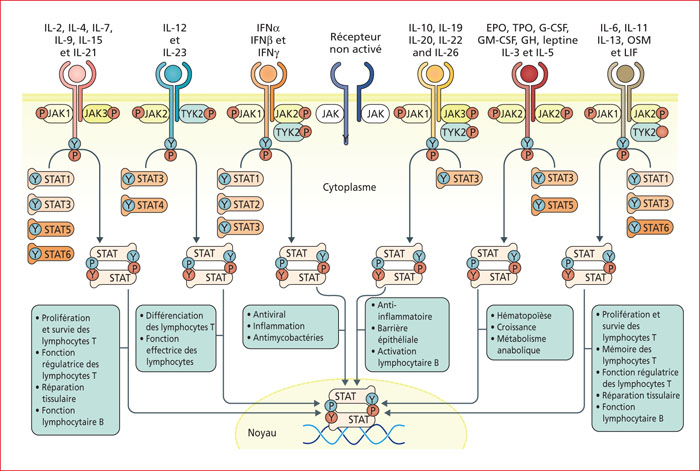
The JAK-STAT pathway is involved in many biological mechanisms, within multiple cell types. An optimal function of the JAK-STAT pathway in hematopoietic and non-hemopoietic cells of the intestinal mucosa is crucial to maintain a homeostatic intestinal barrier. Several dysfunctions of the JAK-STAT pathway have been linked to intestinal inflammation and Inflammatory Bowel Diseases (IBD). As compared to usual anti-cytokine treatments, JAK inhibitors have the theoretical advantage to inhibit simultaneously the production of several cytokines. As the JAK-STAT pathway is complex, the precise mechanism of action of JAK inhibitors remains unclear. While JAK inhibitors are designed to be selective or non-selective, even selective JAK inhibitors impact all JAK molecules due to the high homology of JAKs (JAK1, JAK2, JAK3, TYK2). Tofacitinib, a pan-JAK inhibitor, is currently the only JAK inhibitor with FDA's and EMA's approvals to treat ulcerative colitis (UC). Several other JAK inhibitors are in various stages of development in Crohn's disease and/or UC. The phase III trial, SELECTION, recently showed that filgotinib, a JAK1 selective inhibitor, was efficient to induce and maintain remission in UC. There is a significantly increased risk of herpes zoster associated with tofacitnib. This risk seems less with JAK1 selective inhibitors. As several cases of thrombo-embolic diseases with tofacitinib (10 mg twice daily) in Rheumatoid Arthritis have been reported, it is not recommended to use tofactinib (10 mg twice daily) for maintenance treatment in patients with thrombo-embolic risk factors and therapeutic alternatives.