Hépato-Gastro & Oncologie Digestive
MENUInduction chemotherapy in locally advanced rectal cancer: a new paradigm? Volume 21, issue 6, Juin 2014
- Key words: neoadjuvant chemotherapy, locally advanced rectal cancer, PRODIGE 23
- DOI : 10.1684/hpg.2014.1031
- Page(s) : 439-47
- Published in: 2014
Locally advanced rectal adenocarcinomas are frequent and remain of poor prognosis. Improvements in treatments have mainly focused on increased local control of locally advanced rectal cancer using concomitant radio-chemotherapy followed by surgery with total excision of the mesorectum. The local control is obtained for more than 90% of the patients. But at 5 years follow up, 25% to 30% present a metastatic failure, cause of death of majority of the patients. The intensification of preoperative radiochemotherapy regimens has resulted in increased toxicities, without survival benefit.
The goal of a neoadjuvant chemotherapy should be to reduce the risk of metastatic spread by early delivering of optimal doses of chemotherapy, then preventing the metastatic seeding before the curative treatment of the primary by irradiation and surgery.
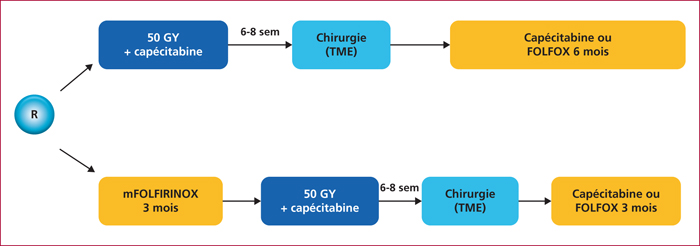
The rational of such a strategy is presented, and a focus is made on the ongoing phase III trial named PRODIGE 23/ACCORD 23. In this study, a neoadjuvant chemotherapy consisting on mFOLFIRINOX during 3 months, followed by a radio-chemotherapy delivering 50 Gy in 25 fractions and capecitabine as radiosensitiser, compared to an upfront radiochemotherapy followed after surgery by adjuvant chemotherapy. This experimental neoadjuvant schedule should be delivered only within clinical trials, the standard preoperative treatment remaining chemoradiotherapy.