Hépato-Gastro & Oncologie Digestive
MENUEverolimus and sunitinib in digestive neuroendocrine tumours: practical use Volume 24, supplement 3, Novembre 2017
Figures
Tables
service de gastroentérologie et pancréatologie,
DHU UNITY,
100 Boulevard du Général Leclerc,
92110 Clichy, France
Clichy, France
- Key words: targeted therapies, neuroendocrine tumours, side effects, sunitinib, everolimus
- DOI : 10.1684/hpg.2017.1545
- Page(s) : 22-38
- Published in: 2017
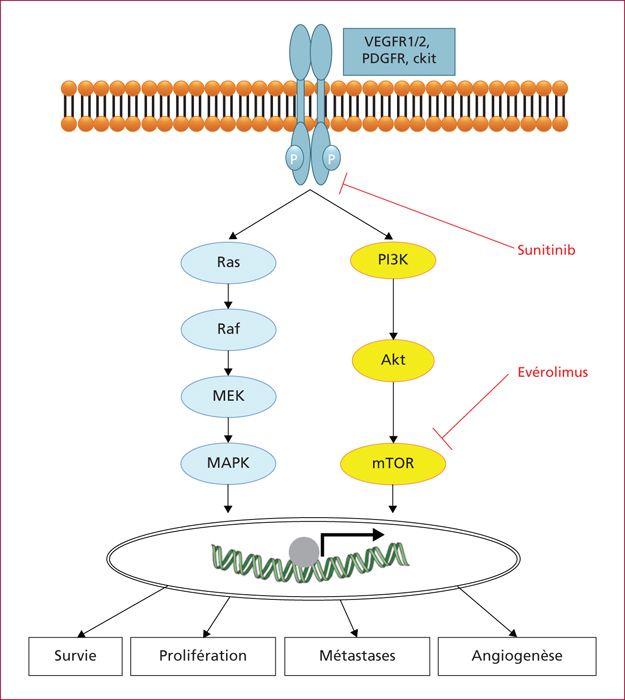
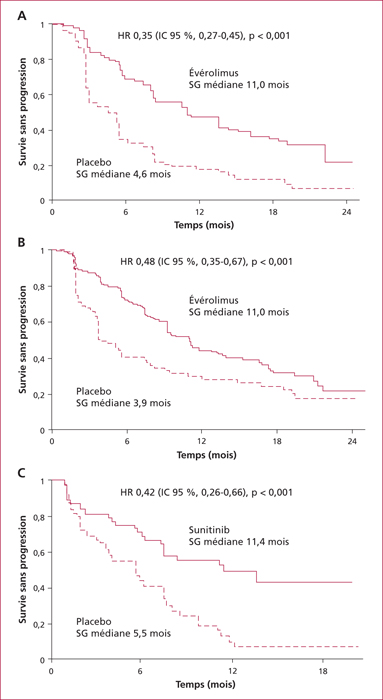
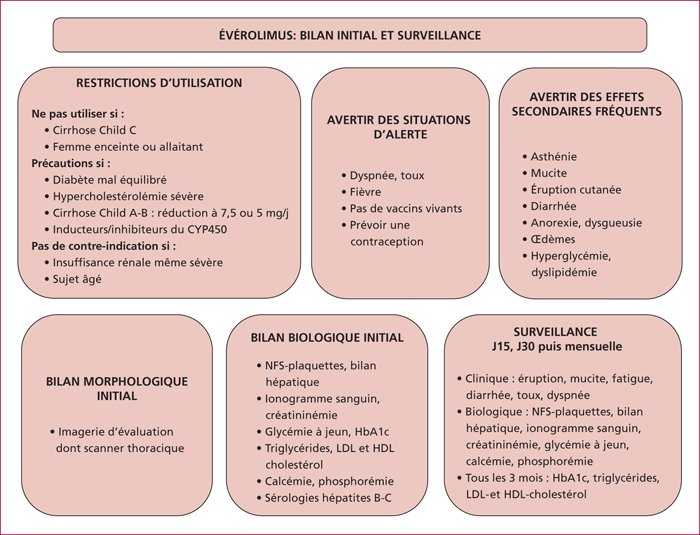
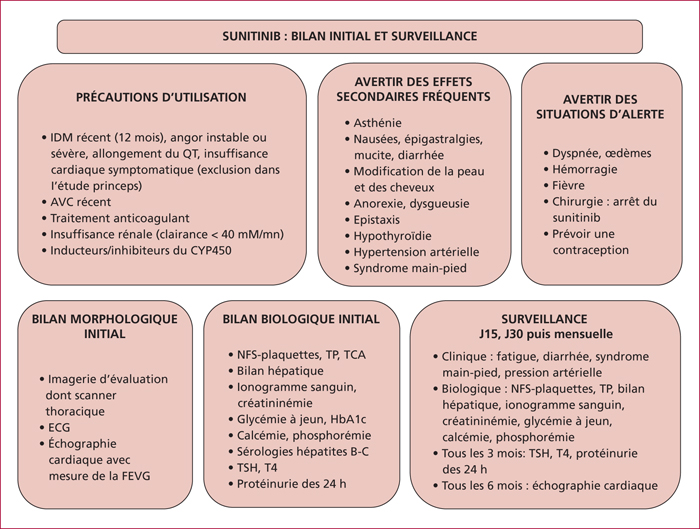
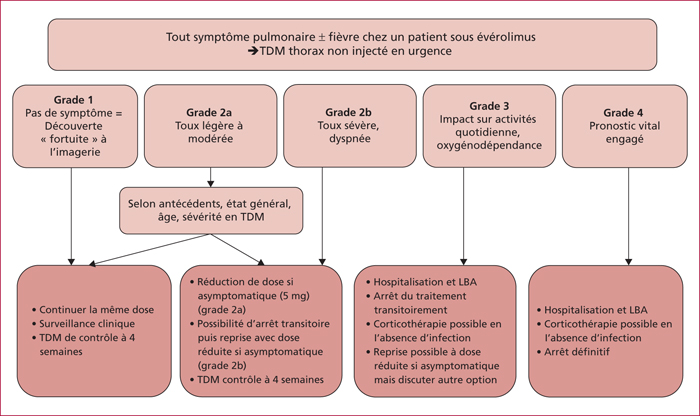
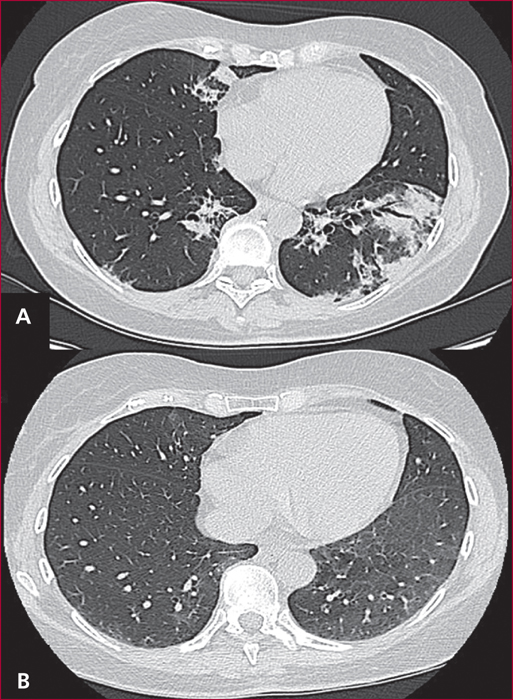
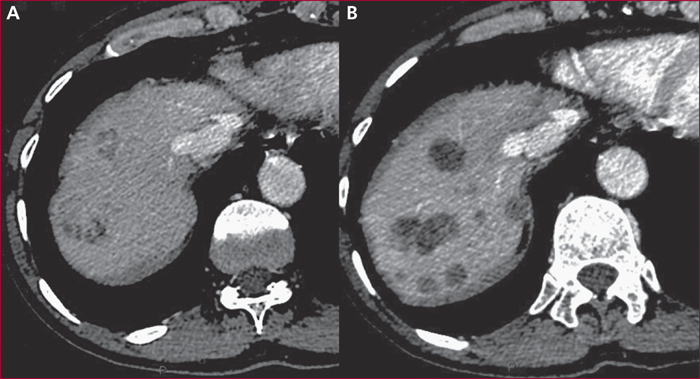
Everolimus inhibits mTOR, a key actor of cell metabolism, proliferation, survival and tumour spread. Sunitinib inhibits tyrosine-kinase receptors involved in neoangiogenesis and tumour survival and growth. They are indicated in the treatment of progressive and metastatic well-differentiated pancreatic (and intestinal for everolimus) neuroendocrine tumours. Any association with inhibitors or inductors of cytochrome P450-3A4 must be avoided, or with dose adaptation. Everolimus and sunitinib have specific side effects. Generally speaking, the occurrence of mild or severe adverse events imply to reduce the drug dose by 25 to 50% or to stop the targeted therapy, respectively. Everolimus-related most frequent adverse events include stomatitis (64%), skin rash (38%), diarrhoea (33%) and asthenia (31%). Severe side effects can occur in up to 46% of patients. Everolimus can also induce hyperglycaemia and hypercholesterolemia that must be managed. Pneumonitis can occur in up to 17% of patients and can be severe and life-threatening in 2 to 8 % of patients. Sunitinib-related most frequent adverse events include diarrhoea (59%), nausea (4%), asthenia (34%), neutropenia (29%) and high blood pressure (26%). A sunitinib-related cardiovascular toxicity is rare but potentially severe. Overall, the choice between these two targeted therapies must depend on patient individual comorbidities and the expected tolerance profile. Most toxicities are manageable easily, although patient education is primordial to optimize observance and improve quality of life.