Hématologie
MENUTreatment in 2022 of hairy-cell leukemia and the variant form Volume 28, issue 2, March-April 2022
- DOI : 10.1684/hma.2022.1751
- Page(s) : 116-30
- Published in: 2022
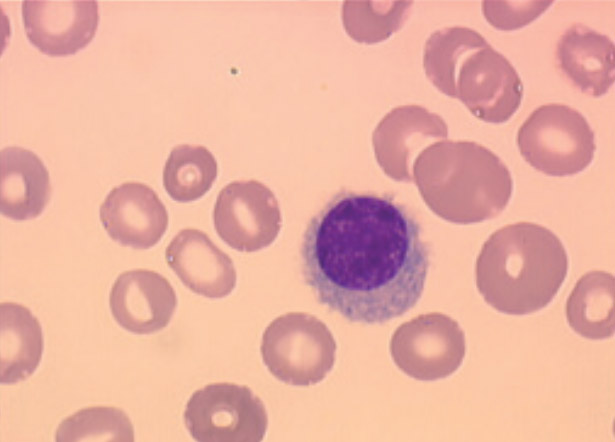
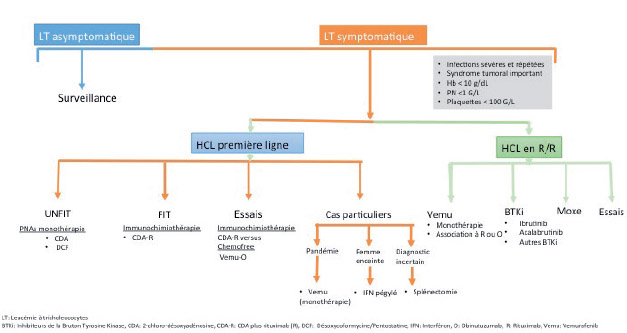
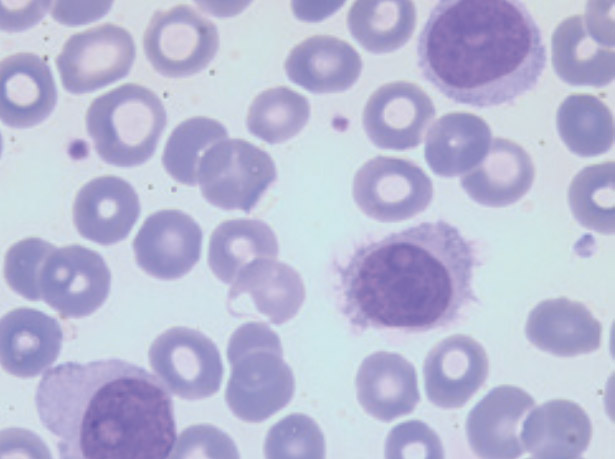
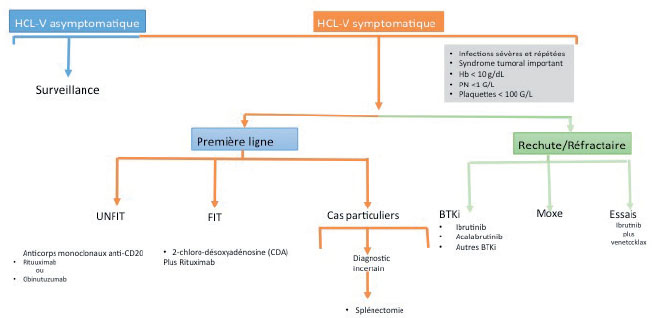
Hairy cell leukemia (LT/HCL) accounts for 2% of all leukemias. The diagnosis is based on the presence in the blood and/or the marrow of hairy cells: abnormal B lymphoid cells with a hairy cytoplasm expressing CD103, CD123, CD11c and CD25. The BRAFV600E mutation of the proto-oncogenic B-Raf gene (BRAF), a molecular marker of the disease, is present in more than 80 % of cases. LT should be distinguished from other chronic B lymphoproliferative syndromes and in particular from other hairy cell proliferations including the variant form of hairy cell leukemia (LT-V). Progress has recently been made in the management of HCL patients. Nucleoside purine analogs (PNAs) in monotherapy either deoxycoformycin or 2-chloro-deoxyadenosine remain the first-line option of treatment. PNAs associated with anti-CD20 monoclonal antibodies (rituximab, obinutuzumab) are now being introduced as the first-line tratment in young and fit patients. The immunochemotherapy allows to obtain prolonged complete remissions. In patients with relapsed/refractory HCL, new therapeutic options: immunotoxins, BRAF inhibitors (BRAFi) or Bruton Tyrosine Kinase inhibitors (BTKi) have to be discussed. In patients with LT-V who do not have the BRAFV600E mutation but mutations in mitotogen-activated protein kinase kinase 1 (MAP2K1) in about a third of cases, immunochemotherapy has become the standard of first-line tratment. In all cases, prolonged haematological monitoring is necessary, because of the increased risk of secondary cancer and in particular that of malignant hematologic disorders. Complex cases are to be discussed in a national multidisciplinary consultation meeting, which will be implemented very soon.