Hématologie
MENUInherited Factor XIII deficiency: Diagnosis, prevalence and treatment modalities in 2020 Volume 26, issue 4, Juillet-Août 2020
Figures
- Key words: Factor XIII (FXIII), bleeding, diagnostic, treatment
- DOI : 10.1684/hma.2020.1571
- Page(s) : 192-200
- Published in: 2020
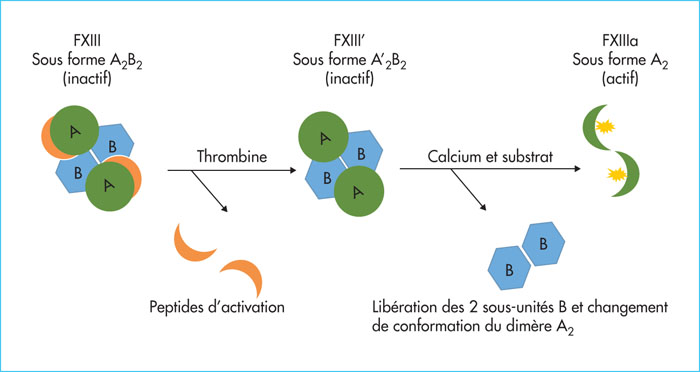
Congenital factor XIII (FXIII) deficiencies account for 6% of rare bleeding disorders in France. FXIII is a protein that plays two roles: 1) It increases clot strength at the very end of the coagulation cascade; 2) it maintains pregnancy. Some 80% of severe forms are revealed by abnormal bleeding when the umbilical cord separates during the neonatal period, and 30 % by spontaneous intracranial hemorrhage (ICH). FXIII deficiencies cannot be detected using the standard tests – prothrombin time and partial thromboplastin time – since FXIII involvement begins after initial clot formation. A FXIII functional activity assay is recommended for diagnosis, and a FXII antigen assay for the classification of FXIII deficiency. FXIII-A antigen assays display excellent correlation with functional measures as well as a very low detection threshold less than 4 %. Their automation therefore enables specific measurement of FXIII, which is indicated when tell-tale clinical signs are observed. In France, the treatment is based on the administration of plasma-derived FXIII concentrates (Fibrogammin®) whose half-life is 11 to 14 days. In cases of acute hemorrhage, in particular intracerebral hemorrhage, the initial dose should be between 20 and 40 IU/kg. Due to the high risk of ICH, the recommendation is to initiate prophylaxis replament therapy as soon as the diagnosis has been made. The recommended dose and dosing interval are at least 20-40 IU/kg once every 4 weeks.
![]() This work is licensed under a
Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License
This work is licensed under a
Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License