Epileptic Disorders
MENUUnder-reporting of nocturnal seizures using video-based home monitoring: a case study on the evaluation of the effect of vagal nerve stimulation Volume 20, issue 6, December 2018
A reduction in self-reported seizures is the standard measure of efficacy of interventions for refractory epilepsy. While there have been major advances in treatment alternatives for this patient group, such as new antiepileptic drugs (AEDs), neuromodulation (vagus nerve stimulation (VNS)), and deep brain stimulation (DBS), few advances have been made to objectively and accurately document treatment response. This is most likely of great importance as seizure under-reporting is a recognised issue that can considerably affect the outcome of treatment. Inaccurate documentation of seizures depends mostly on the seizure type, but documentation accuracy also varies in individual patients from month to month (Cook et al., 2013). On average, fewer than 50% of seizures are documented (Elger and Hoppe, 2018), but documentation rates as low as 15% of the true seizure count of nocturnal seizures have been reported (Hoppe et al., 2007).
Here, we present a case study highlighting the magnitude and significance of under-reporting seizures, with particular reference to the evaluation of treatment response to VNS and the type of patient accommodation (home vs. a special-needs boarding school).
Case study
The patient is a 17-year-old girl with moderate intellectual disability and focal refractory epilepsy due to bilateral polymicrogyria. She had suffered from recurrent seizures despite trials of various AEDs since the age of two. She is currently treated with 750 mg BID oxcarbazepine, 150 mg BID zonisamide, and 20 mg OD clobazam. As the patient was not a candidate for resective surgery, she was scheduled for VNS surgery in October 2017. In order to assess a baseline for nocturnal seizures before operation, she underwent video recording in May 2017. The recording was performed at the patient's home with a video monitoring system provided by Neuro Event Labs. The system consisted of a single wall-mounted infrared 1080p camera, with optical analysis software designed to detect nocturnal seizure events. The camera had a remote control which allowed initiation and termination of the recording, limiting the recording periods according to the patient's wishes. Although the system is capable of detecting seizure-like behaviour automatically, the recordings were watched in their entirety in order to ensure that no seizures were missed.
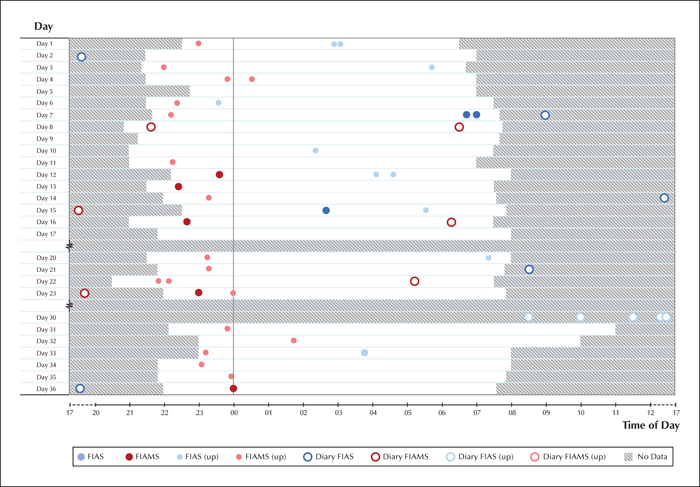
Registered seizures were compared to findings from a video-electroencephalography (vEEG) performed in December 2016, in order to verify the suspected epileptic seizures. The seizures were classified according to the recent ILAE guidelines (Fisher et al., 2017). Two distinct seizure types were captured on the vEEG (see video sequence):
- –Seizure typeI.B.01. A focal impaired awareness motor seizure (FIAMS), with the following seizure components: sudden swinging of the upper extremities; followed by complete flexion of the body; slight, fast shaking of the arms; and a high-pitched voice. This was the predominant seizure type based on vEEG and was the only one that the parents detected during the night.
- –Seizure typeI.B.08. A focal impaired awareness seizure (FIAS), with the following seizure components: eye opening; followed by a silent, slow turning of the head and eye deviation to the right; clenching of the hands with slight dystonic posturing of the wrists; and stiffening of the trunk. Postictally, the patient presented with slow blinking and lip smacking. In contrast to the motor seizure, this seizure type was recognized by the parents only during the daytime, as the patient collapses due to impaired awareness. In addition to these two seizure types, unpropagated forms of these seizures occurred; these appeared much like the first few seconds of the complete seizures, but ceased before progression to a prolonged state of unawareness.
All of the aforementioned components were required to be present during the home recordings in order to classify events according to seizure type. Twenty-seven nights were recorded over a 36-day period, totalling 276 hours of video. This task was performed by a medical student (SP), who compared the findings to vEEG, marking all possible seizures as events and defining the start and end of the clinical manifestation of the seizures. The epileptic nature of these events was confirmed by two epilepsy experts (JP and SLH), and further subdivided into corresponding seizure types. In cases of disagreement, the events were re-evaluated in collaboration in order to come to a consensus.
VNS was successfully implanted in October 2017, and active stimulation was commenced two weeks after the operation. A target stimulation current of 1.75 mA (frequency: 30 Hz; pulse width: 250 μsec; on-time: 30 seconds; off-time: 5 minutes; autostimulation parameters were the same as in normal mode stimulation) was reached in January 2018, without any intolerable side effects. The AEDs were not altered after implantation.
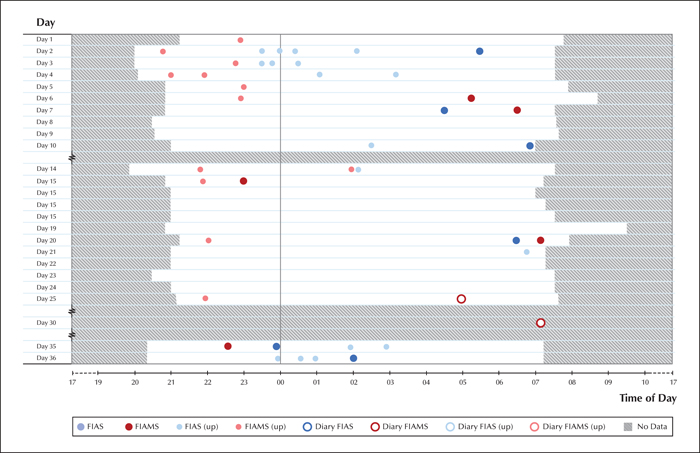
A new monitoring period was scheduled from the end of January until the end of February. The recordings were carried out with a similar setup as for the initial registration, with the exception that the patient was staying in a boarding school, rather than at home.
Seizure types were categorized as before, and a professional vEEG nurse (AJ) inspected the entire recording corresponding to 24 nights over a 36-day period, totalling 260 hours of video, marking all potential seizures as events. These were then classified by experts (JP and SLH), as before (figures 1, 2).
The frequency of seizures noted on a seizure diary versus video, before and after VNS implantation, is presented in table 1, as mean values over a 28-day period. Seizure-free days were considered only when video was recorded during the preceding night. While there was a significant decrease in overall seizure count based on diary-based recordings, there was a slight increase in fully propagated seizures and a major increase in the number of focal impaired awareness seizures, relative to the video recording. It is notable that for all categories, actual seizures were heavily under-reported in the diary.
Discussion
The present case emphasizes the importance of an objective, non-human-based seizure detection system for accurate follow-up of patients within the context of treatment intervention. Without the use of the objective seizure registration system, the effect of VNS would have been interpreted in a completely different way, which would not have accurately reflected the impact of the therapy on seizure frequency and duration. In addition, without objective seizure registration, the change in accommodation (transition from home to a boarding school environment) may have been a source for bias in the assessment of seizure frequency post-VNS.
Our patient underwent video monitoring before and after VNS implantation, the latter taking place in a boarding school. The results provide some insight into the true effects of neuromodulatory intervention, compared to baseline, and also demonstrate the intraindividual variation that may be generated using a subjective seizure diary. Over the 28-day period, based on video monitoring, the average seizure count of the predominant seizure type and its unpropagated form decreased by 26% following VNS therapy, whereas the corresponding diary-based recording indicated a false decrease of 80.6%. The less-disabling FIASs and their unpropagated forms led to more initial disagreement between the two experts classifying the seizures, indicating a difficulty in separating seizures with a low motor component from regular sleep movement. On the other hand, these seizures had increased in frequency by 12.1%, which further emphasizes the importance of evaluating all seizure types.
The number of seizure-free days increased more than three-fold from an average of 3.1 to 10.5 over the 28 days. The change in seizure-free nights may also be a clinically meaningful effect of VNS therapy that can be objectively evaluated with the video-based monitoring system. Only nocturnal seizures followed a similar pattern of change, with motor seizures decreasing and FIASs increasing in frequency.
Another notable difference was reported regarding the accuracy of caregivers to detect and register seizures (when the patient was incapable of doing so). In the first setting, the patient was almost continuously supervised by a parent sleeping on the floor next to her, whereas in the boarding school, nocturnal surveillance was not continuous and occurred at random intervals. The recognition rate of all nocturnal motor seizures was 14.8% (4/27 seizures) in the home environment and 5.6% (1/18 seizures) in the institutional environment. When taking only the fully propagated seizures into account, the trend persists: 100% (4/4 seizures) accuracy in seizure recognition at home and 20% (1/5 seizures) accuracy in the institutional environment. The diary count for the other seizure type, FIAS, could not be calculated as this type was not recognized by the caregivers during the night.
The more accurate diaries, however, may take their toll. Parents are more likely to share a room with their child with epilepsy than with healthy siblings. This, along with the disease of the child, has a negative effect on the caregivers’ quality of life (QoL). Caregivers are therefore more likely to have a worse quality of sleep, more parasomnias, and sleep disturbances, resulting in more exhaustion and daytime dysfunction than those without a sick child (Larson et al., 2012). This can lead to reduced vitality and mental health (van Andel et al., 2009). Some of the burden caused to caregivers could be alleviated with the installation of a camera-monitoring system, diminishing caregivers’ fears of not observing seizures.
The system used in our case study was a marker-free video detection system, the type of which has been proven to be accurate for seizures with motor components. These systems can have sensitivities of up to 97%, with a mean false detection rate (FDR) of 0.78 per night for tonic-clonic seizures (Geertsema et al., 2018) and a specificity and sensitivity of above 85% when distinguishing between focal clonic seizures, myoclonic seizures, and random movement in infants (Karayiannis et al., 2006). At the lowest, the sensitivity can be only 57% (Geertsema et al., 2018) and the specificity just 53% (Lu et al., 2013). Our method, based on visual confirmation of the seizures by human experts, hopefully provides more accurate results.
Despite being a useful patient follow-up tool, video recording-based systems have their disadvantages. A marker-free method provides greater convenience for the patient, but may make it more challenging to detect seizure-related motion. The camera must be placed so that the patient is in view. Visibility can often be impaired by blankets or an adverse position of the body. An obvious problem is also privacy of patients, however, patients can interrupt recording on demand. In addition, the system is limited to only highly intense, large seizures (clonic, hypermotor), excluding those with subtle motor, sensory or autonomic symptoms, thus rendering it useful for only a particular portion of those suffering from epilepsy.
In conclusion, our case emphasizes the inconsistencies in patient-based seizure reporting. An objective detection system can improve the accuracy of seizure diaries in some cases, giving a more realistic view of treatment efficacy and potentially enhancing patient care. This can also reveal changes in seizure pattern, reduction, and severity, providing information otherwise unattainable for the patient and their caregiver. The extent and clinical significance of our findings based on a single patient will need to be addressed in future studies using a large patient cohort with similar methodology.
Supplementary data
Summary didactic slides are available on the www.epilepticdisorders.com website.
Acknowledgements and disclosures
The authors would like to thank Asta Junttari, Tatiana Anagnostaki and Anna Hakala for their contributions towards the project. SP works as a medical consultant for Neuro Event Labs. AK and JP are founders and shareholders of Neuro Event Labs. MDA is an employee of LivaNova PLC, manufacturer of vagus nerve stimulators, and holds stock options. SLH and SR do not have any conflict of interest to declare in relation to this paper. The contents of this article are based on the authors’ research and do not necessarily represent the official view of Neuro Event Labs.
* The images of this manuscript have been presented at meetings organized by Neuro Event Labs for doctors, mostly neurologists, in order to demonstrate the usability of their system. The manuscript itself has not been presented elsewhere.