Epileptic Disorders
MENUMusicogenic and spontaneous seizures: EEG analyses with hippocampal depth electrodes Volume 16, issue 4, December 2014
Reflex seizures (RSs) can be triggered by a wide variety of external or internal stimuli. Triggers such as flashing lights or visual patterns, somatosensory stimulation, reading, eating, listening to music, thinking, playing chess, answering the telephone, brushing teeth, and exposure to hot water have been reported in the literature (Goossens et al., 1990; Wieser et al., 1997; Michelucci et al., 2004; Zifkin and Inoue, 2004; Ferlazzo et al., 2005; Pittau et al., 2008; Striano et al., 2012; Kumada et al., 2013). The majority of RSs have been already identified on the basis of the stimulus that can cause them.
Musicogenic seizures (MS) are triggered by listening to music in most cases, but playing, thinking, or dreaming of music have all been reported (Shaw and Hill, 1947; Dearman and Smith, 1965; Sutherling et al., 1980; Zifkin and Zatorre, 1998; Avanzini, 2003). The type of music varies largely from one patient to another, and classical, religious, popular, military, sad, and sentimental kinds of music are reported as provoking factors. In these cases, seizures following a musical stimulus can often be delayed by several minutes. During this latent period, patients may experience distress, agitation, tachycardia, and rapid breathing building up to the seizure. An affective or emotional content of the music is often described in patients with MS (Koelsch, 2005). However, frequently, the effective stimulus appears to be very complex, associating strong emotional feelings with specific sensorial and perceptive factors and requiring a long exposure to the effective stimulus.
Musicogenic seizures investigated by scalp EEG and neuroimaging studies have indicated that multiple regions are involved. However, usually involvement of the temporal regions, with a right-sided preponderance, is reported (Tayah et al., 2006; Mehta et al., 2009; Duanyu et al., 2010). The role of the right temporal lobe (TL) was emphasized in only a few investigations with intracranial EEG monitoring (Tayah et al., 2006; Mehta et al., 2009; Duanyu et al., 2010). Spontaneous seizures are also reported in some cases (Wieser, 1998; Tayah et al., 2006; Pittau et al., 2008).
Although spontaneous seizures and complex seizures that occur independently of any external stimuli, such as dreaming and thinking, are described for patients with MS, musicogenic epilepsy is not classified under the 2001 ILAE classification as a specific syndrome (Engel, 2001).
Here, we present a patient with MS from whom invasive recordings were obtained using subdural arrays, as well as hippocampal depth electrodes. Interestingly, this patient had both spontaneous seizures and MS (box 1), and they originated from different hippocampi.
Case study
A 33-year-old female was admitted because of intractable seizures since the age of 17 years. Typically, she had seizures characterized by an aura consisting of a boring sensation and oral automatisms with impairment of consciousness. For the previous eleven years, since getting divorced, her seizures were mostly precipitated by listening to affective music in her native language (Turkish). She did not describe the occurrence of seizures in non-musical emotional situations. Carbamazepine, valproic acid, oxcarbazepine, levetiracetam, and pregabalin failed to control the seizures.
She had a history of a febrile convulsion. Her family history was unremarkable.
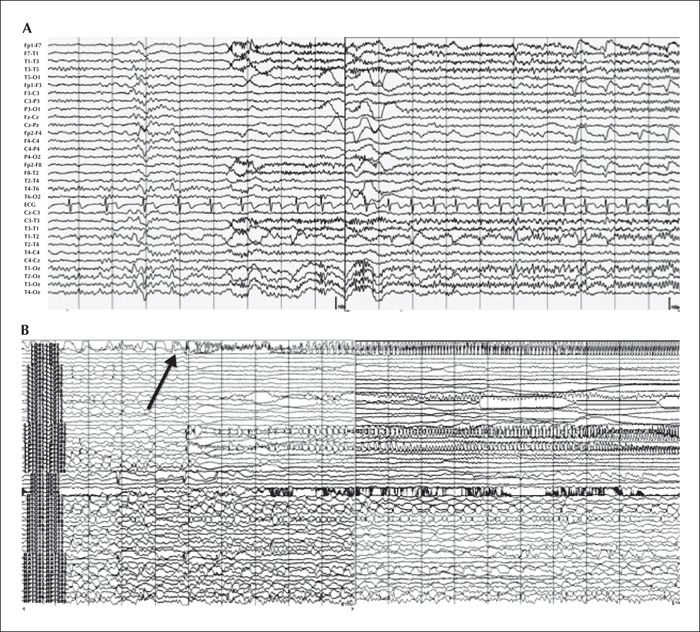
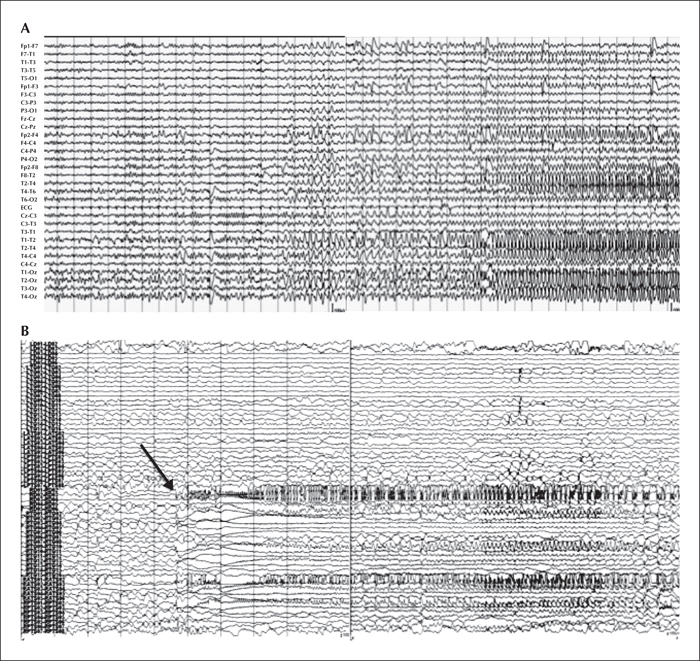
During video-EEG monitoring, seven seizures were recorded, which showed left (n=2) and right TL (n=5) EEG seizure onset. All seizures with left TL onset occurred spontaneously, during sleep (figure 1A). She could not describe the aura in these left-sided spontaneous seizures. All five seizures with right TL onset were precipitated by listening to emotional melodies. Listening to classical, neutral, and affective music in foreign languages for 10-15 minutes did not provoke seizures. All right-sided seizures were recorded within 2-5 minutes of listening to sentimental/emotional music in her native language (figure 2A). Her auras were boring sensations in some of these MS. She could not easily recognize or differentiate those spontaneous seizures or MS. Interictal epileptiform discharges were recorded almost equally in the right and left temporal regions.
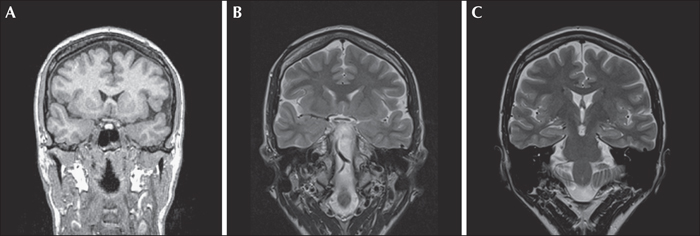
High-resolution brain magnetic resonance imaging (MRI) was obtained using a 1.5 T scanner (Symphony, Siemens, Erlangen, Germany). An MRI protocol for weighted (W) magnetization-prepared gradient-echo (MPRAGE) imaging was obtained parallel to the brainstem, and T2-W turbo spin-echo (TSE) and T1-W inversion in routine brain imaging. Her cranial MRI showed mild atrophy of the left anterior TL (figure 3A, B). Both hippocampi were normal in terms of thickness, signal intensity, and internal structure (figure 3C). Her cranial fluorodeoxyglucose positron emission tomography (PET) revealed hypometabolism in the right TL. Her neuropsychological examination revealed moderate bilateral hippocampal dysfunction for memory. There was no lateralized hippocampal dysfunction, leading to a decision of temporal lobectomy with good cognitive function during the postoperative period. To further investigate the presence of unilateral and serious hippocampal dysfunction, we performed the Wada test. The Wada test revealed better memory performance with the left hippocampus. She had good performance in free recall (>60%) and recognition (>90%) during testing of the left hippocampus. But these ratios were 20% and 60%, respectively, for right hippocampal functions.
Her invasive EEG monitoring was also performed with subdural electrodes covering both lateral and mesial TL, including both hippocampal depth electrodes. She had both spontaneous seizures and habitual MS. Similar to her scalp EEG, spontaneous seizures originated from the left hippocampus (figure 1B) and MS started from the right hippocampus (figure 2B). During MS, the ictal onsets occurred first as high-frequency activity at the first three contacts of the right hippocampal depth electrodes and propagated to the right mesial temporal strip electrodes within 1-2 seconds. After 3-5 seconds, the seizures spread to the grid electrodes overlying the right lateral temporal cortex. During spontaneous seizures, the ictal EEG onsets occurred at the first two contacts of the left hippocampal depth electrodes and propagated to the mesial temporal electrodes in a few seconds. The spread to the left lateral temporal cortex occurred 7-10 seconds later.
Due to the bilateral ictal onset findings, she was not eligible for surgical resection. She was advised to avoid the triggering type of music, but this was not possible. Therefore, vagal nerve stimulation (VNS) treatment was considered. After this VNS surgery, in addition, she received 3,000 mg/day levetiracetam and 800 mg/day carbamazepine treatment; she had only three seizures in the first year and was seizure-free during the following year.
Discussion
In this case report, we recorded MS precipitated by emotional music in the patient's native language, originating from the right hippocampus. Interestingly, she also had spontaneous seizures recorded from the left hippocampus. We imagine that the emotion and early memories combined to precipitate MS related to the right hippocampus.
Invasive EEG recordings in patients with MS are very rare. Accordingly, subdural recordings from one patient with the presenting symptom of tinnitus revealed a focus from a cortical dysplasia in the superior temporal gyrus (Duanyu et al., 2010). In the second report, Tayah et al. (2006) showed foci in both TLs. Similar to our patient, EEG recordings with depth electrodes were reported in only one patient with ictal onset in the right hippocampus (Mehta et al., 2009). Compared to these cases, we recorded spontaneous seizures in the left hippocampus in addition to right-sided MS complicated with emotion and early memories.
The PET study of our patient also revealed right-sided abnormality. The results of functional imaging, PET or ictal single-photon emission computed tomography (SPECT) in patients with MS mostly demonstrated similar findings with right TL involvement (Wieser et al., 1997; Gelisse et al., 2003; Tayah et al., 2006; Mehta et al., 2009; Pittau et al., 2008). The results of these investigations are valuable for patients with non-lesional MRI, such as our patient.
At present, the exact pathophysiology of MS is undetermined. Hyperexcitable cortical areas can be stimulated to different degrees and extents by different musical stimuli. Musical processing encompasses brain mechanisms in musical perception, recognition, and emotion. Musical perception requires the decoding of a musical stimulus within the primary auditory cortex in Heschl's gyrus and the association cortex in the superior temporal gyrus. The primary auditory cortex is thought to receive thalamic afferents from the medial geniculate nucleus, which in turn connect through networks to the association cortex, mesolimbic systems, and other multisensory cortices (Stewart et al., 2006). Musical recognition and emotion are thought to involve orbitofrontal areas and the limbic system, which may serve to store past auditory memory and emotional evaluation of a musical stimulus (Dellacherie et al., 2009). In our case, music could create an emotional state that was responsible for seizure precipitation (Koelsch, 2005). This complex network activity within the primary auditory cortex and association cortex may project to the hippocampus and amygdala where the memory and emotional component of the music are encoded. The emotion and memory triggered by music are suggested as a causal factor of MS, rather than the auditory content of the music. Although we had no depth electrodes in lateral TLs including primary auditory and association cortices, the ictal onsets of our patient's MS were not recorded with subdural electrodes on the lateral part of the TL. Furthermore, the latency between exposure to the music and the clinical seizure onset of 2-5 minutes must be evidence that the epileptogenic area did not primarily involve the auditory cortex. The first ictal EEG changes were recorded from right hippocampal depth and mesial temporal strip electrodes. Recording of ictal onset with right hippocampal depth electrodes, with strong feelings of worries related to early memories at the beginning of seizures, supports the emotional trigger effect in MS.
Although she had left-sided spontaneous seizures, affective music only provoked seizures in the right hippocampus. Furthermore, she had no right- or left-sided seizures in non-musical emotional situations, such as conversations involving emotional memories or looking at old photographs. The specificity of the right hippocampus for the emotional component of sad musical stimuli may be considered. Patients with right amygdala damage showed enhanced memory for emotional stimuli, although their overall memory performance was reduced (Buchanan et al., 2001; Mitterschiffthaler et al., 2007). Our patient also had poor memory function related to the right hippocampus, but she had enhanced emotional memories during the MS originating from there. Patients with right-sided damage can show a normal pattern of facilitation of memory related to emotional music.
In neuroradiological studies with functional MRI, in patients with left TLE, a decrease in cerebral activity associated with a decrease in emotional word activity, with respect to neutral word activity, was observed (Richardson et al., 2004). Similar to these results, our patient had no emotional component in seizures originating from the left side.
Surgical treatment should be considered, especially in patients with intractable seizures with unilateral focal ictal onsets. However, bilateral independent MS or independent spontaneous seizures in addition to MS, as in our patient, preclude surgery. VNS treatment was almost successful in our patient and can thus be considered in patients with bilateral or independent MS, with or without spontaneous seizures.
Acknowledgements and disclosures
The authors have no conflicts of interests to disclose.