Epileptic Disorders
MENULingual epilepsia partialis continua: a detailed video-EEG and neuroimaging study Volume 22, issue 4, August 2020
Motor epilepsia partialis continua (EPC) of the tongue is an extremely unusual epileptic condition. In recent years, a few clinical cases, some containing video images, have been reported (Kinirons et al., 2006; Nayak et al., 2006; Vukadinovic et al., 2007; Li et al., 2010; Yilmaz et al., 2010; Iyer et al., 2014; Sureshbabu et al., 2017). However, in most descriptions, the ictal recordings were either normal or inconspicuous.
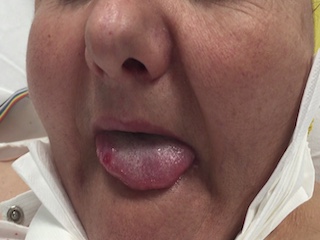
We present a case of lingual EPC secondary to low-grade glioma in which the EEG and neuroimaging features were particularly remarkable.
Case study
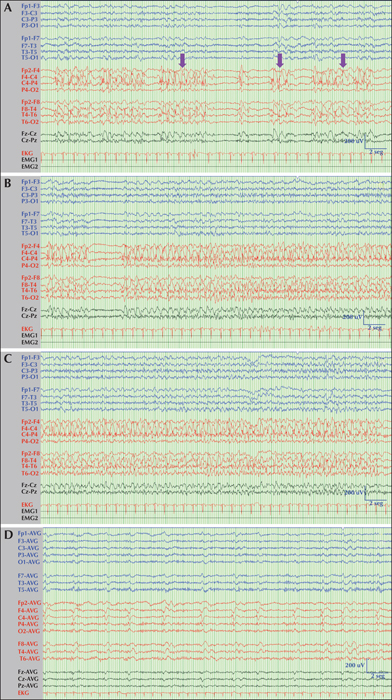
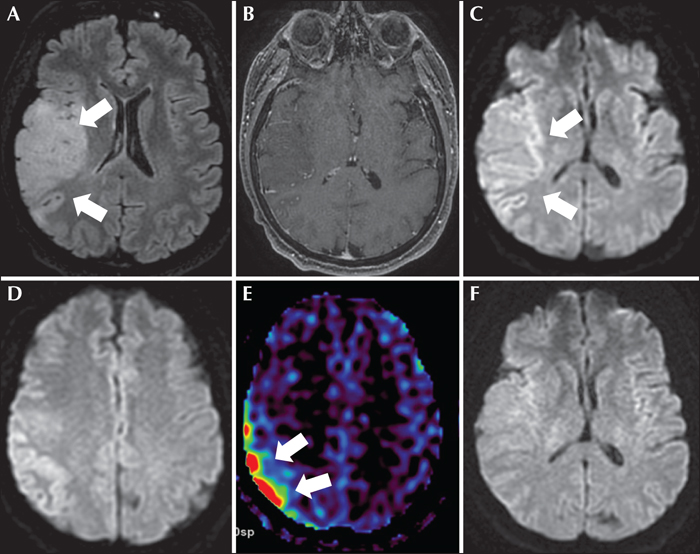
A 61-year-old woman with a diagnosis of mild cognitive impairment and anxious-depressive disorder was admitted to a local hospital due to left hemifacial clonic movements and dysarthria. Her mental state was normal and no other neurological signs or deficits were observed. A computed tomography (CT) scan of the brain was normal. Subsequently, she experienced a generalized tonic-clonic seizure and an episode of postictal agitation. Treatment with intravenous levetiracetam (1,000 mg/24 h) was started, and the patient was transferred to our Neurology Unit. Upon arrival, she was aware and agitated but no focal motor or sensory deficits were seen. During examination, her speech was slurred and dysarthric. Routine laboratory tests and cerebrospinal fluid were within normal limits, and a second brain CT scan was unremarkable. Subsequently, the episodes of left facial jerks and dysarthria recurred for several hours and phenytoin (100 mg/8 h) was added to her antiseizure drugs (ASDs). A video-electroencephalogram (v-EEG) showed lateralized periodic discharges with superimposed rhythmic activity (LPDs-plus) that occurred every two seconds with stereotyped appearance affecting a wide area of the right hemisphere that included the frontal and temporal lobes (figure 1). These periodic discharges occurred in mixed clusters conforming to diplopleds and polypleds (Reiher et al., 1991). These clusters progressively increased in duration during the recording. Moreover, we captured four partial motor seizures consisting of tongue jerks, mandibular trismus, inability to speak (anarthria) and hypersalivation (see figure 1 and video sequence). These episodes lasted for approximately one minute. There was no involvement of palatal or pharyngeal muscles innervated by brainstem motor neurons. The patient did not refer to any sensory (numbness, tingling) symptoms preceding the onset of motor twitches. She tried voluntarily to control her motor manifestations, but tongue clonic movements remained invariable. Ictal and interictal epileptiform discharges were maximal at frontal (C4, F4) and temporal (F8, T4) contacts. In short, these findings were compatible with the diagnosis of partial motor status epilepticus or motor EPC arising from the right hemisphere. Brain magnetic resonance imaging (MRI) showed a right temporo-insular cortico-subcortical lesion, hyperintense on FLAIR, suggestive of low-grade glioma (figure 2). In addition, diffusion-weighted imaging (DWI) and an arterial spin labelling (ASL) series showed restricted diffusion in the right temporo-insular and parietal cortex and increased cerebral flow, respectively (figure 2). All these findings were in keeping with changes related to persistent status epilepticus. In view of the persistence of the seizures, lacosamide (200 mg/12 h) was added to her ASD therapy. Three days later, seizures were controlled and a second v-EEG revealed continuous LPDs proper occurring every two seconds involving the right frontal and temporal lobes (figure 1). The patient was assessed by our neurosurgeons who recommended to perform a control MRI and follow-up in the outpatient clinic. Meanwhile, she was being treated with phenytoin (350 mg/24 h), lacosamide (200 mg/24 h) and levetiracetam (2000 mg/24 h) with only partial control of her seizures since she experienced occasional motor and complex partial seizures with secondary generalization. A second brain MRI performed one month later showed persistence of the tumour without restricted diffusion or increased blood flow. In view of this clinical evolution, the patient accepted to undergo an immediate surgical treatment. However, two days later, she suffered a cardiorespiratory arrest and died.
Discussion
In this report, we describe a case of lingual EPC secondary to low-grade glioma in which v-EEG showed LPDs-plus and frequent recurrent focal epileptic seizures, which have not previously been documented for this entity. In addition, we carried out a complete neuroimaging evaluation that included FLAIR, DWI and ASL sequences.
Lingual EPC occurs rarely. This epileptic condition has been observed in the setting of focal (Rasmussen, herpes virus simplex) or paraneoplastic encephalitis, neurocysticercosis, vascular malformations and cerebral tumours (table 1). However, the EEG and neuroimaging findings observed in our patient have not been reported before. In most previous descriptions, the ictal recordings were either normal or inconspicuous (table 1). By contrast, the EEG of the patient described here was notably abnormal and helped us to establish an accurate diagnosis of lingual EPC. LPDs-plus is widely considered a peri-ictal phenomenon, often occurring very close to seizures. This is a good example of the ictal-interictal continuum, in which LPDs disappear during the seizures and reappear in the postictal period, building a subtle continuum between the interictal, ictal and postictal states. Additional electrophysiological studies such as somatosensory evoked potentials, long-loop reflexes and back-averaging techniques may be helpful to confirm the diagnosis or for pathophysiological analysis of motor EPC (Fernández-Torre et al., 2004; Bien and Elger, 2008). However, in our case, we did not carry out further investigations because ictal v-EEG and neuroimaging were sufficient to establish the diagnosis.
In addition, neuroimaging findings were markedly striking. DWI MRI revealed restricted diffusion in the right temporo-insular and parietal cortex, and ASL series showed an increased cerebral flow that was compatible with changes associated with status epilepticus. Previous studies have already highlighted the usefulness of DWI imaging for detecting the vascular and metabolic alterations occurring during status epilepticus and LPDs on EEG (Di Bonaventura et al., 2009; Giovannini et al., 2018). As in our case, restricted diffusion and hyperintensity on T2-weighted and FLAIR sequences, with data of increased perfusion, are the most frequently encountered alterations. These findings represent a mixture of vasogenic and cytotoxic oedema.
We believe that the symptoms reflect an activation of the primary motor cortex (motor homunculus) although DWI and ASL sequences showed abnormalities involving the right temporoinsular and parietal cortex. This finding could indicate the participation of different networks involved in the motor function. Indeed, the parietal cortex (Brodman areas 5 and 7) is part of the motor program and receives essential sensory information to perform voluntary movement. Therefore, it is not unexpected that the neuroimaging revealed parietal changes. Moreover, epileptiform discharges affected a large frontal and temporal area (maximum over the central and mid-temporal regions) that may also account for these alterations.
The report by Jabbari and Coker (1981) merits further discussion, as the authors designed a study to identify epileptiform movements of oropharyngeal muscles in 86 chronic epileptic patients. They found rhythmic movements of the tongue in three children. These movements affected only the tongue and occurred mainly during sleep. The authors attributed this to an unusual type of subcortical seizure as the movements were associated with episodic changes (desynchronized sleep) on the EEG. Polysomnography was not performed. Of note, Lu et al. (2005) reported that in the rat, a species widely used to study the neural mechanisms of both sleep and breathing, lingual electromyographic activity is minimal or absent during slow-wave sleep and then gradually increases after the onset of rapid eye movement sleep due to the appearance of large phasic bursts. Indeed, phasic tongue movements in human rapid eye movement sleep have been observed (Chokroverty, 1980). It is worth mentioning that in the three cases, tongue movements occurred associated with a desynchronized EEG and abolition of the spike-wave complexes. Therefore, it is possible that the movements of the tongue described in this study were not of epileptic origin, as the authors in fact recognized at the end of their article. In any case, the patient described here corresponds to a completely different clinical and EEG scenario and for that reason we have not included this article in table 1.
In clinical practice, neurologists rarely bear in mind that lingual EPC can be a cause of language impairment. Early ictal speech has been well-described in fronto-mesial epileptic seizures. Indeed, in the case described by Meletti et al. (2003), the inability to move the tongue was always associated with speech arrest, suggesting an ictal disruption of motor execution (motor inhibition). It is important to bear in mind that the supplementary motor area is one of the main components of the central motor program (Fernández-Torre et al., 2019). However, in the reported case here, the speech disorder (most frequently dysarthria and occasionally anarthria) was one of the most important clinical manifestations, probably associated with the inability to move the tongue via muscle contractions. This finding is not new and in the past other authors have highlighted this symptom. Kinirons et al. (2006) described a patient who was unable to speak and, on occasion, was dysarthric. In addition, Iyer et al. (2014) reported a five-year-old boy with mild dysarthria and swallowing difficulties accompanying his involuntary tongue movements.
Clinically, an isolated non-epileptic lingual myoclonus should be included in the differential diagnosis in these cases. We can distinguish two types of non-epileptic lingual myoclonus: one that is secondary to a lesion of the nervous system (symptomatic lingual myoclonus), most likely due to brainstem damage in the context of Arnold-Chiari malformation, pontine traumatism or hypoxia, and another in which a specific cause is not found which is called “essential lingual myoclonus” (Bettoni et al., 1999; Patel and Tappuni, 2018). Obviously, the use of v-EEG and brain MRI are necessary for reaching an accurate diagnosis.
In summary, we describe a case of lingual EPC in which we observed LPDs-plus, recurrent focal seizures and neuroimaging findings of vasogenic and cytotoxic oedema that have not previously been reported. Our case report contributes to expanding the spectrum of this rare epileptic entity.
Disclosures
None of the authors have any conflict of interest to declare.