Epileptic Disorders
MENUEpileptic diaphragm myoclonus Volume 14, issue 4, December 2012
epd.2012.0540
Auteur(s) : Andreas Hahn andreas.hahn@paediat.med.uni-giessen.de, Bernd A Neubauer
Department of Neuropediatrics, University of Giessen, Giessen, Germany
Correspondence. Andreas Hahn Department of Neuropediatrics, Feulgenstr 10-12, 35392 Giessen, Germany
Singultus is caused by a myoclonic contraction of the diaphragm and other inspiratory muscles which is followed by glottal closure about 35 milliseconds later, thereby producing the characteristic “hiccup” sound. While acute singultus is a frequent, self-limiting, and benign phenomenon, chronic hiccupping represents a rare and potentially debilitating condition occasionally caused by central nervous system abnormalities (Loft and Ward, 1992).
Epilepsy has only rarely been suspected or described to be related to hiccupping (Lin et al., 1998, Fogarasi et al., 2006; Ponnusamy et al., 2008). We report, for the first time, a child with epilepsy and singultus as the main seizure manifestation. Polygraphic ictal recordings, performed to elucidate the underlying electrophysiological mechanisms, suggest that the diaphragm myoclonus was caused by central activation of the polysynaptic hiccup reflex arc.
Case Report
A 6-year-old girl was referred to our department due to a first photic-induced generalised seizure. She had been walking with her mother along an alley while the sun was shining through the trees. Both were watching the clouds, when the girl suddenly stopped and starred with fluttering of her eye lids. She then sat down on the ground and began to jerk rhythmically for two minutes while falling onto her left side. On admission, her neurological status was normal. The family and the girl's history were uneventful, but the mother mentioned the occurrence of brief vocalisations of the girl several times per day that started about three months ago. These episodes were particularly frequent when watching television and had been interpreted by the parents as an expression of excitement.
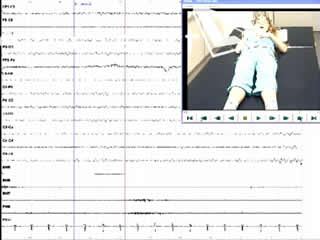
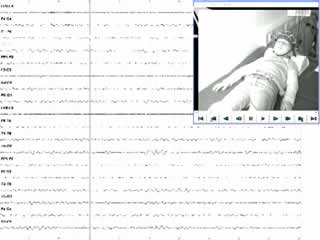
A routine video-EEG showed normal background activity, bilateral, synchronous generalised spike-waves at rest, and marked photosensitivity. During hyperventilation and drowsiness, several brief generalised spike-wave discharges of 2.5-3/second, associated with a prominent sobbing sound during inspiration and a visible contraction of the diaphragm, were registered (video sequences 1 and 2). These episodes were assumed to be hiccup seizures.
Because of the exceptional seizure symptomatology, a video-polygraphic recording, including a total of 15 minutes of hyperventilation and a period of sleep, was performed on the following day. The EEG signal (bandpass: 0.5-120 Hz) was registered by surface electrodes placed on the scalp, according to the international 10-20 system. Surface EMG signals were recorded simultaneously using the belly-tendon technique; from the right and left deltoids and wrist extensors, and from the right and left diaphragm (Demoule et al., 2003). The EEG signals were acquired by means of a referential montage and EEG and EMG signals were digitised using a sampling frequency of 512 Hz. All ictal recordings were visually evaluated using an expanded time scale and an appropriate amplitude display.
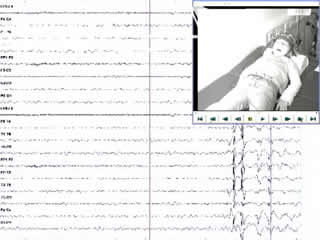
Nine ictal epochs, seven occurring during hyperventilation, were registered and used for analysis. Slow motion video analysis suggested that the sobbing sound was produced at the end of the inspiratory phase of the respiratory cycle. During seizures, EMG discharges from the right and left diaphragm lasting 104-131 milliseconds, but not from the deltoid and wrist extensor muscles, were consistently recorded (video sequence 3). Each EMG burst was temporally related to a double-spike-and-slow-wave complex and maximum at frontal contacts on EEG. The initial EEG spike preceded the onset of the EMG discharge by 56-64 milliseconds (median: 59 milliseconds), the first positive wave by 27-39 milliseconds (median: 31 milliseconds), and the second spike by 9-14 milliseconds (median: 10.5 milliseconds) (figures 1 and 2). No hiccup seizures were recorded during photic stimulation.
Additional examinations including high-resolution brain MRI, visually and somato-sensory evoked potentials, and transcranial magnetic stimulation gave normal results. The girl became seizure-free by taking 500 mg valproate per day.
Discussion
Myoclonus is characterised by brief, shock-like jerks that may be generalised and confined to large or small groups of muscle, or even restricted to a single muscle. Myoclonus is termed “epileptic” when occurring in combination with cortical epileptiform discharges (Commission, 1997). In our patient, a classification of epileptic myoclonus was based on the consistent temporal relationship between the spike-wave discharges and the diaphragmatic EMG bursts.
Singultus has only rarely been reported as a seizure symptom (Lin et al., 1998; Fogarasi et al., 2006; Ponnusamy et al., 2008). Lin et al. (1998) noticed brief expiratory vocalisations during seizures in six children with benign myoclonic epilepsy of infancy and speculated that contractions of the diaphragm contributed to these noises, however, since the diaphragm functions purely during inspiration (Demoule et al., 2003), this seems implausible. Fogarasi and co-workers (2006) briefly mentioned hiccupping as an autonomic symptom in a child with temporal lobe epilepsy, yet the patient's complete seizure symptomatology was not given. Recently, Ponnusamy et al. (2008) reported on a girl with absence epilepsy who had hiccups during one of her absences.
Our case differs from those reported by Fogarasi et al. (2006) and Ponnusamy et al. (2008), since hiccupping constituted the major seizure symptom and could be provoked repeatedly by hyperventilation. Abnormal EEG findings together with normal development prior to epilepsy, normal neurological status, and absence of structural brain pathology are features of a primary generalised form of idiopathic epilepsy. However, in contrast to ictal polygraphic recordings of other forms of idiopathic myoclonic epilepsies, which have been reported to demonstrate bilateral synchronous jerks of the upper limbs or generalised myoclonia (Panzica et al., 2001; Guerrini et al., 2005), EMG discharges in our case remained limited to the diaphragm.
In juvenile myoclonic epilepsy, jerk-locked back-averaging revealed positive-negative EEG transients preceding EMG bursts of the deltoid muscles by about 10.5 milliseconds (Panzica et al., 2001), which is consistent with myoclonic activity descending from the sensorimotor cortex through the rapid conduction pyramidal pathways (Panzica et al., 2001; Guerrini et al., 2005). In contrast, latencies between spike-wave complexes and deltoid EMG potentials were found to range from 21 to 80 milliseconds in 9 children with various types of myoclonic epilepsy, suggesting polysynaptic impulse transmission (Oguni et al., 1997). In young healthy adults, the latencies of motor evoked potentials elicited by transcranial magnetic stimulation and recorded by abdominal surface electrodes from the diaphragm were found to range from 15.6 to 20.4 milliseconds (Demoule et al., 2003). In our patient, the intervals between the initial spike and the first positive wave of the epileptic discharges, as well as the myoclonic jerks, argue against a monosynaptic impulse transmission, while the short latency of the second spike makes it unlikely that the diaphragmatic EMG bursts were related to this component. Confinement of the myoclonus to the diaphragm is consonant with a central activation of the hiccup reflex arc by the epileptic activity.
The hiccup reflex consists of an afferent portion (vagus nerve, phrenic nerve, and sympathetic chain T6-T12), an efferent pathway (phrenic nerve), and a less well defined central part (Loft and Ward, 1992). A putative primary hiccup centre has been localised in the medulla oblongata, but persistent singultus caused by brain pathology in the hypothalamus, reticular activating system, and temporal lobe demonstrates additional modulation by supratentorial structures (Loft and Ward, 1992; Ponnusamy et al., 2008). This shows that activation or irritation of the reflex arc may occur at different levels in the CNS. Although the majority of ictal hiccups in our patient were recorded during hyperventilation, this correlation does not seem sufficient enough to allow a classification of reflex ictal phenomenon triggered by forced respiration. Ponnusamy et al. (2008) hypothesized that ictal hiccups in their case were caused by abnormal thalamocortical excitation underlying absence seizures. Whether these mechanisms are also crucial in our patient remains speculative.
Disclosures
The authors have no conflicts of interests to declare.
Legends for video sequences 1 Video sequence 1 Recording of three hiccup seizures during hyperventilation. 2 Video sequence 2 Recording of two hiccup seizures during drowsiness. 3 Video sequence 3 Polygraphic recording of three hiccup seizures. Lanes 5 and 6 are recordings from the left and right deltoid muscles, and lanes 7 and 8 are recordings from the left and right diaphragm, respectively. 4 Key words for video research on www.epilepticdisorders.com Syndrome: idiopathic generalized not specified Etiology: genetic disorder Phenomenology: hiccup; myoclonic seizure; Localization: not applicable