Epileptic Disorders
MENUAtonic seizures in children with surgically remediable epilepsy: a motor system seizure phenotype? Volume 19, issue 3, September 2017
Atonic seizures are common in several electroclinical epileptic syndromes that begin in infancy or early childhood, such as Dravet syndrome, epilepsy with myoclonic atonic seizures, and Lennox-Gastaut syndrome. Negative motor phenomena may be encountered in atypical benign epilepsy with centrotemporal spikes. However, atonic seizures are also observed in epilepsy with focal seizures of structural aetiology. Whereas focal atonic phenomena characterized by ictal limb or facial paresis, or negative myoclonus are well described in this setting (Abou-Khalil et al., 1995; Noachtar and Lüders, 1999; Matsumoto et al., 2005; O’Donovan et al., 2007), only few video-EEG documentations of focal seizures with axial or generalized ictal atonia are available (Satow et al., 2002; You et al., 2007; Zhao et al., 2010; Kovac et al., 2012). The latter phenotype might be difficult to recognize as a focal seizure if the precise electroclinical characteristics are lacking. These aspects are particularly relevant in early-onset pharmacoresistant epilepsies associated with MRI-obscure cortical developmental malformations.
The pathophysiological basis of ictal atonia is still under debate. In seizures with focal atonic semiology, there is growing evidence for a predominant location of the epileptogenic areas within the frontal or peri-central part of the cortical motor system (Tinuper et al., 1998; Noachtar and Lüders, 1999; Matsumoto et al., 2000; Rubboli and Tassinari, 2006; Kovac et al., 2012; Maillard et al., 2014). Alteration in electrical activity within the distinct areas of dorsolateral or mesial associative motor cortex, defined as primary and supplementary negative motor areas (Lim et al., 1994; Lüders et al., 1995), or within the primary motor or sensory cortex (Ikeda et al., 2000; Matsumoto et al., 2000; Kovac et al., 2012), may produce focal atonic signs. Moreover, the frequency content of the ictal discharge within the primary motor cortex could be important for the semiological expression of ictal phenomenon as positive or negative motor signs (Maillard et al., 2014). As for generalized atonia, the few available human intracerebral EEG studies have analysed cases of epilepsy with hypothalamic hamartoma and demonstrated that atonic seizures in this particular context were associated with extended bilateral frontal cortical discharges (Munari et al., 1995; Kahane et al., 2003). An acute perturbation in the corticoreticulospinal regulatory pathway has been hypothesized as a possible mechanism underlying a sudden loss of postural tone, being a hallmark of some generalized epileptic syndromes (Elger et al., 1981; Mori et al., 1995; So, 1995; Kovac and Diehl, 2012).
Our objectives were to assess the electroclinical phenotype of atonic seizures in surgically remediable paediatric patients and to study the organization of the underlying epileptogenic zone defined by intracerebral EEG (icEEG) recordings.
Methods
Patients and EEG recordings
In this retrospective study, we analysed two consecutive, serially evaluated paediatric patients presenting with atonic seizures as a manifestation of pharmacoresistant epilepsy of structural aetiology, as evidenced by scalp- and stereotactic intracerebral video-EEG-recordings (SEEG). Both patients underwent presurgical assessment, followed by resective surgery at the Epilepsy Unit of the Strasbourg University Hospital (France). They were identified from 114 intracerebral EEG investigations performed at our unit between 2004 and 2015.
We used the ILAE definition of atonic seizures (Blume et al., 2001) to distinguish seizures that are characterized by a sudden loss or diminution of muscle tone without apparent preceding myoclonic or tonic event, involving the head, trunk, jaw, or limb musculature.
Presurgical evaluation included detailed clinical history, physical examination, long-term video/scalp-EEG recordings according to the 10-20 system with simultaneous bipolar surface EMG recording from the right and left deltoid muscles, neuropsychological evaluation, high-resolution 1.5 Tesla MRI, and metabolic imaging using FDG-PET.
Both patients required icEEG to allow more precise definition of the epileptogenic zone (EZ) and subsequent tailored resection. SEEG recordings were performed at the Epilepsy Unit of the Strasbourg University Hospital. Intracerebral multiple contact electrodes (5-18 contacts of 2 mm length; diameter: 0.8 mm; 1.5 mm apart; Dixi Medical, France) were implanted unilaterally in different cortical areas, using a frameless stereotactic surgical robot ROSA™. The placement of electrodes was guided in each patient by the hypotheses for EZ localization, based on available non-invasive data. The frontal, insular, and temporal regions were explored in the first patient and the frontal and insular regions in the second patient. A cranial CT scan was performed after intracerebral electrode implantation to verify the absence of any complication and the spatial accuracy of the implantation. 3D CT/MRI data fusion was used for the accurate anatomical localization of each electrode contact.
Long-term scalp and icEEG recordings were performed using a DeltamedTM system (SEEG signal sampling rate: 256 Hz; high-pass filter cut-off: 0.16 Hz).
Both families gave their written informed consent prior to investigation.
Seizure analysis
For each patient, scalp video-EEG and SEEG recordings of all seizures were reviewed. The clinical duration and the topography of the muscle atonia (generalized or segmental), the presence of other clinical ictal phenomena, as well as the order of appearance of atonic semiology during the seizure were analysed.
In this retrospective study, the polygraphic monitoring, performed within the frame of a routine presurgical work-up, was limited to bilateral EMG recordings from the deltoid muscles in Patient 1 and additionally covered the neck muscles in Patient 2. However, the ILAE definition of atonic seizures does not formally require an extended polygraphic documentation. The ictal atonia definition was based on clinical observation of loss of posture on video recording, as well as on absence of myographic activity demonstrated on the EMG traces. The atonic phase comprised the whole period of muscle atonia, with absence of visible voluntary movements, including the fall period. Total seizure duration was defined as the period between the electrographic seizure onset and its termination.
Analysis of anatomo-electroclinical organization of the epileptogenic zone
The hypothesis for the localization of the EZ was made based upon icEEG and neuroimaging data. For the purpose of the anatomo-electroclinical correlations, the boundaries and subdivisions of dorsolateral (lateral Brodmann area 6 [BA6] and Brodmann area 8 [BA8]) and mesial (supplementary motor area [SMA], pre-supplementary motor area [preSMA], and cingulate motor area [CMA]) premotor cortex were defined according to previous proposals (Rizzolatti et al., 1998; Matsumoto et al., 2007; Bonini et al., 2013). The primary motor (MI) and the primary sensory (SI) areas were defined according to cortical electrical stimulations, performed as a part of the routine pre-surgical SEEG evaluation.
We determined a quantitative definition of the EZ based on the estimation of a mathematical quantity referred to as the “epileptogenicity index” (EI) in brain structures, involved (or not) with the ictal discharge. Epileptogenicity index is a novel quantitative measure that characterizes the epileptogenicity of brain structures recorded by depth electrodes, with the aim of objectively quantifying and defining a neuronal network underlying seizure generation. In this method, each recorded SEEG signal is analysed in the transition period from interictal to ictal activity and two parameters are evaluated and combined into a single quantity:
- –the propensity of a given brain structure to generate rapid discharges (beta [12.4-24 Hz] and gamma [24-90 Hz] bands), considered as a typical electrophysiological pattern of the EZ in focal epilepsies, replacing background activity (delta [1-3.4 Hz], theta [3.4-7.4 Hz], and alpha [7.4-12.4 Hz]);
- –the delay of appearance of this rapid discharge in a given structure with respect to the first structure, itself involved in a “rapid discharge mode” (Bartolomei et al., 2008a).
In this respect, the EI is built on the same information as that used during visual review of EEG seizure recordings. In practice, we use a semi-automatic approach involving a handy graphical user interface; the user can easily inspect and validate automatically-detected change points indicating the accurate onset of rapid discharges. From this validation performed on a “structure-by-structure” basis, the EI is then computed.
The EI estimation can be used as a classification measure to distinguish between epileptogenic and non-epileptogenic structures independently of the underlying pathological substrate or the patient's age (Aubert et al., 2009; Bartolomei et al., 2011; Bonini et al., 2013; Sevy et al., 2014). The method has been recently validated in the setting of epileptogenic neurodevelopmental disorders (de la Vaissière et al., 2014; Gollwitzer et al., 2016; Lagarde et al., 2016).
A normalized EI value is used, ranging from 0 to 1. If there is no involvement of the brain structure, EI=0 (no epileptogenicity), whereas if the brain structure generates a rapid discharge and the time to seizure onset is minimal, EI=1 (maximal epileptogenicity). An EI between 0 and 1 corresponds to secondary involvement of the brain structure concerned (for detailed methodology, see Bartolomei et al. [2008a]). We arbitrarily set a cut-off EI value, i.e. a value above which we considered a structure as highly epileptogenic and thus belonging to the epileptogenic zone, as EI>0.4. This cut-off was defined as optimal based on a previous study on epileptogenicity of developmental lesions (Aubert et al., 2009), where this value corresponded to the 90th percentile of EI values in the intrinsically epileptogenic lesional sites. EI values from brain structures investigated were computed and averaged for all invasively recorded seizures in each patient.
Results
Clinical data
The patients were aged 6 months and 36 months, respectively, at epilepsy onset. Atonic seizures were first reported at age 16 months and 36 months, respectively. Both patients had uneventful personal history with uncomplicated pregnancy and birth. Neither had a family history of epilepsy. Neuropsychological evaluation demonstrated moderate developmental delay (Patient 1) or deficits in executive functions and working memory (Patient 2), which installed after epilepsy onset. Clinical data are summarized in table 1.
Scalp electroclinical findings
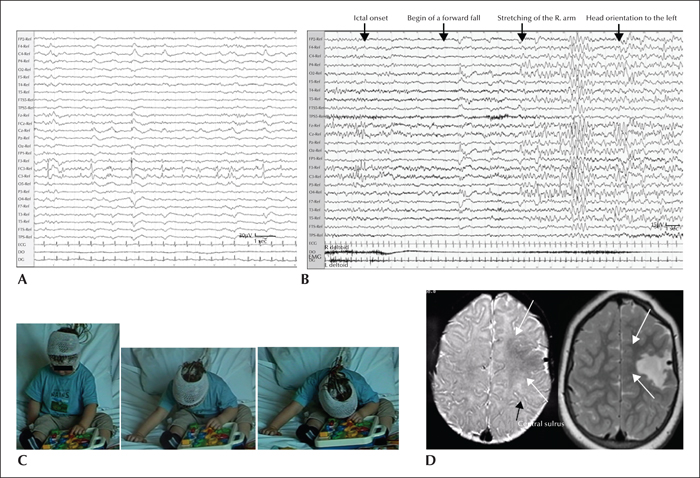
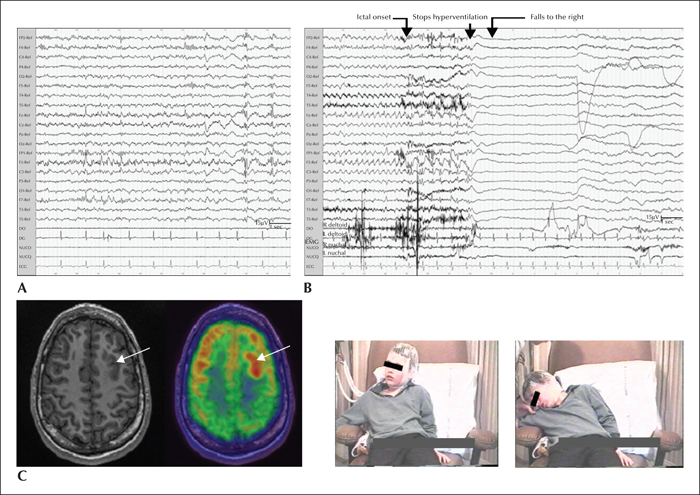
In Patient 1, interictal EEG showed focal pseudorhythmic epileptiform discharges and slowing over left precentral and vertex regions, with presence of recurrent bursts of low-voltage fast activity of the same topography (figure 1A). This periodic pattern increased and became continuous during non-REM sleep, suggesting a characteristic surface EEG aspect of focal cortical dysplasia (FCD) type 2 (Palmini et al., 1995; Chassoux et al., 2000; Tassi et al., 2012). In Patient 2, scalp EEG showed both regional (bilateral frontal) and generalized interictal epileptiform discharges (figure 2A). Generalized polyspike bursts were sometimes symptomatic of the myoclonia of the isolated head, face, or arms. This patient was initially misdiagnosed with progressive myoclonic epilepsy.
A total of nine atonic seizures (3-6 per patient) were analysed. The negative motor semiology comprised sudden generalized atonia with dropping of the head and arms and loss of postural tone, leading to a fall. The duration of the atonic phase was 47.5±21.0 seconds in Patient 1 and 23.6±3.2 seconds in Patient 2. Representing more than 70% of the total seizure duration (63.8±13.1 seconds and 30.0±1.7 seconds, respectively), it was the core but not the only ictal phenomenon. In both patients, the atonic phase was preceded by a brief behavioural arrest. In Patient 1, a subtle concomitant “positive motor” sign (slight stretching of the contralateral arm) was observed during the atonic phase, followed by contralateral dystonic arm posturing and contralateral head orientation.
The ictal EEG onset patterns were immediately bilateral, predominant over frontal regions (Patient 2; figure 2B), or localized over a precentral region on the lesion side with fast bilateral spread (Patient 1; figure 1B).
Neuroimaging findings
In Patient 1, MRI displayed an extended lesion, suggestive of FCD, located in the dorsolateral premotor region, adjacent to the primary motor cortex (figure 1C). In Patient 2, initial MRI at age 5 years was considered normal. At age 16 years, FDG-PET revealed a well-delimitated hypermetabolic area in the left dorsolateral premotor cortex corresponding to the abnormally deep superior frontal sulcus (figure 2C).
SEEG electroclinical findings
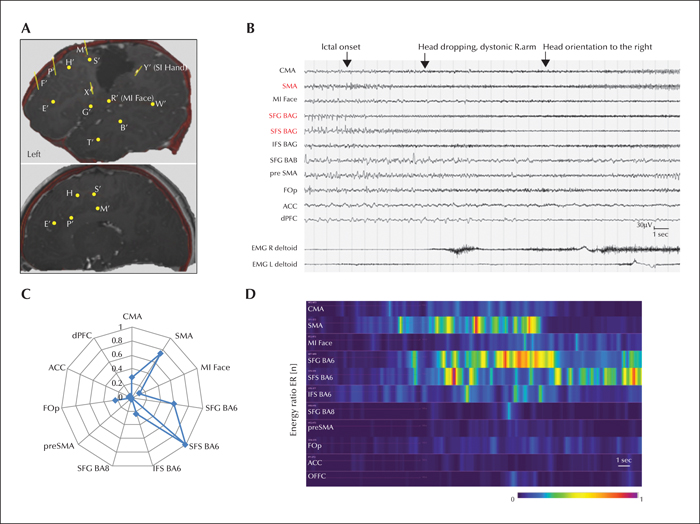
Patient 1 did not become seizure-free after the first cortectomy at age 25 months that targeted the epileptogenic zone, as determined by the MRI lesion and concordant scalp video-EEG. He was investigated using SEEG at age 7 years. Four invasively recorded habitual seizures were analysed. The ictal semiology comprised behavioural arrest, followed by head dropping with dystonic stretching of the right arm, head orientation to the right, and bilateral inferior facial contraction. The duration of this subtle segmental atonic phenomenon was significantly shorter than in infancy: 10.5±2.0 seconds (51% of total seizure duration) versus 47.5±21.0 seconds (75%), respectively. SEEG recordings (figure 3) revealed a mesiolateral premotor epileptogenic network with seizure onset in the lesional dorsolateral premotor cortex (BA6) next to the anterior and superior borders of the first resection, with rapid involvement of the SMA (
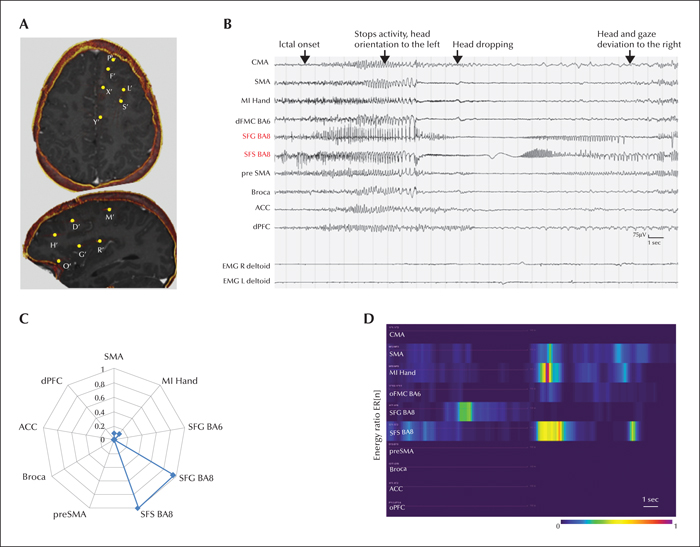
Patient 2 remained misdiagnosed with progressive myoclonic epilepsy until the age of 16 years when a potential structural abnormality was finally suspected on FDG-PET-guided MRI that encouraged subsequent SEEG exploration. Four habitual seizures were analysed. The initial semiology comprised a behavioural arrest, followed by slow head dropping. As for Patient 2, the duration of the negative motor phenomenon became much shorter, compared to the first evaluation: 4.3±1.0 seconds (15% of total seizure duration) versus 23.6±3.2 seconds (79%), respectively. The seizure then evolved with rubefaction and contralateral tonic head and gaze deviation. Ictal SEEG showed seizure initiation within the premotor part of the superior frontal sulcus and rapid implication (figure 4).
In both patients, no isolated atonic phenomena could be elicited by cortical electrical stimulations.
Organization of the epileptogenic zone
The epileptogenicity profiles of the analysed patients are illustrated in figure 5A. The mean EI values of recorded seizures were maximal in the lateral premotor cortex in both patients (0.90±0.15 and 0.78±0.27, respectively). In Patient 1, the quantitatively defined EZ (EI values >0.4) included several lesional and non-lesional structures within the lateral (BA6) and mesial (SMA) premotor cortex (figure 5B). In Patient 2, it was restricted to a part of the lateral premotor cortical area within the “lesional” part of superior frontal sulcus and the adjacent superior frontal gyrus (BA8; figure 5C).
Surgery and outcome
Both patients underwent subsequent tailored resections of the invasively defined epileptogenic zone. Pathological examination of resected specimens showed FCD in both cases. Postoperative seizure outcome corresponded to Engel Class Ia (Engel et al., 1993) at the last follow-up visit. Patient 1 presented with moderate, partially reversible right-sided hemiparesis after the first surgery; no transient or permanent postoperative neurological deficits were reported in Patient 2. Neuropsychological outcome one year after surgery was favourable, with improvement of partial scores (verbal comprehension), but persistence of moderate developmental delay in Patient 1 and normalization of previously deficient scores (backward verbal and spatial working memory spans and cognitive flexibility) in Patient 2.
Discussion
Our study demonstrates that different electroclinical phenotypes might be encountered in focal-onset atonic seizures occurring in infancy or early childhood. The first presented phenotype comprised an ictal atonic phenomenon associated with some lateralizing clinical features and focal scalp EEG abnormalities; the second was characterized by “pure” atonic semiology without lateralizing clinical signs and by the presence of bilateral ictal and interictal EEG patterns. However, long-lasting (over dozens of seconds) generalized atonia was a predominant feature in both cases. This clinical aspect is of particular interest as it corroborates the previously postulated hypothesis that long duration of an atonic phenomenon might be a feature distinguishing the phenotype of focal-onset atonic seizures from that of atonic seizures within the frame of generalized epileptic syndromes, in which brief atonia lasting from milliseconds to 1-2 seconds has been classically reported (Gastaut et al., 1966; So, 1995; Oguni et al., 2001; Satow et al., 2002). Nonetheless, this requires further investigation using a larger patient cohort.
The epileptogenic zone underlying atonic seizures was localized within the premotor regions, rostral or adjacent to the primary motor cortex. Using a quantitative analysis of the intracerebral EEG recordings, we identified a “focal”, dorsolateral premotor EZ in one case and a more distributed mesiolateral premotor EZ in the other case. These findings are in good agreement with previously described motor seizure network subtypes (Bonini et al., 2013). In both situations, the emergence of atonic semiology temporally correlated with nearly simultaneous spread of the tonic ictal discharge to the lateral and mesial premotor and primary motor areas, as demonstrated by SEEG recordings.
According to the recent concept proposed by Bartolomei et al. (2008b), the emergence of clinical signs in a focal seizure depends on the phenomena of synchronized or transitorily desynchronized activity in the cortical structures, defined as the “epileptogenic zone network”, followed by synchronized rhythmic modifications of the other cortical and subcortical structures belonging to the “propagation network”. A recent study based on direct cortical stimulations suggested the possible integrative role of higher-order associative fronto-parietal cortices in producing negative motor signs that would result from impairment of movement organization (Enatsu et al., 2013). Thus, ictal negative motor semiology might not correspond to the involvement of just an isolated brain area (e.g. the so called negative motor areas [Lüders et al., 1995] that would elicit negative motor responses when stimulated), but could reflect a larger perturbation of the cortico-subcortical motor networks.
In the light of these concepts, we hypothesize that atonic seizures might represent a motor system seizure phenotype in the setting of structural epilepsy. A vast initial spread of the high-frequency desynchronizing discharge, arising from the motor areas (whether corresponding to the negative motor area or not), would lead to a functional decoupling between the primary, lower-order, and higher-order associative cortical and subcortical (the fast corticoreticular pathway) parts of the motor system, resulting in disorganization of postural control that underlies generalized atonia. The subsequent decrease in discharge frequency and increase in network synchronization between the specific cortical (the SMA and dorsolateral premotor cortex) and subcortical (the basal ganglia) nodes might mediate the emergence of positive motor signs, such as contralateral tonic/dystonic arm posturing, the M2e posture, or head version (Ajmone-Marsan and Ralston, 1957; Bancaud and Talairach, 1992; Rusu et al., 2005; Bonini et al., 2013; Marashly et al., 2016).
As far as the developmental aspect is concerned, it should be accepted that atonic seizures are not exclusively seen in infants and children, but may also be observed in epilepsy with focal seizures in adulthood. However, in an adult cohort, they were mostly reported as focal and, only exceptionally, as generalized atonia (So, 1995; Kovac and Diehl, 2012). Our findings in two serially evaluated patients suggest a possibility of age-dependent evolution of atonic seizures from prolonged generalized atonia with a few subtle positive motor signs at early age to brief segmental atonia with prominent positive motor signs in later life, along with the maturation of the physiological brain motor networks throughout childhood, adolescence, and early adulthood (Chugani et al., 1987; Chiron et al., 1992; Schäfer et al., 2014).
Finally, our data demonstrate that atonic seizures, commonly associated with generalized syndromes, may have a focal onset and be amenable to surgery. Surgical outcome was excellent in both our cases suggesting that early-onset pharmacoresistant epilepsy manifesting with atonic seizures should be considered for presurgical evaluation if some clinical, EEG or neuroimaging features are in favour of a focal origin.
Conclusion
Long-lasting generalized atonia was a predominant clinical feature of focal-onset atonic seizures occurring in infancy. This ictal semiology evolved into subtle segmental atonia associated with prominent positive motor signs at later age. The spatio-temporal organization of the underlying epileptogenic networks supports the hypothesis that atonic seizures may represent a motor system seizure phenotype in structural epilepsy. Sustained seizure freedom could be achieved by complete removal of the epileptogenic zone.
Acknowledgements and disclosures
We thank Dr Suela Dylgjeri for electrophysiological assessment of the patients investigated. We thank Dr Gabrielle Rudolf (Department of Neurology, University of Strasbourg) for proofreading the manuscript, and Dr Karl Strobl and Dr Thomas Bast (Kork Epilepsy centre) for helpful discussions.
The authors have no conflict of interest to disclose.