Epileptic Disorders
MENUA de novo GABRB2 variant associated with myoclonic status epilepticus and rhythmic high-amplitude delta with superimposed (poly) spikes (RHADS) Volume 22, issue 4, August 2020
- Key words: Alpers syndrome, GABRB2, POLG1, myoclonic status epilepticus, RHADS
- DOI : 10.1684/epd.2020.1183
- Page(s) : 476-81
- Published in: 2020
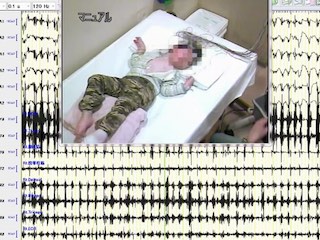
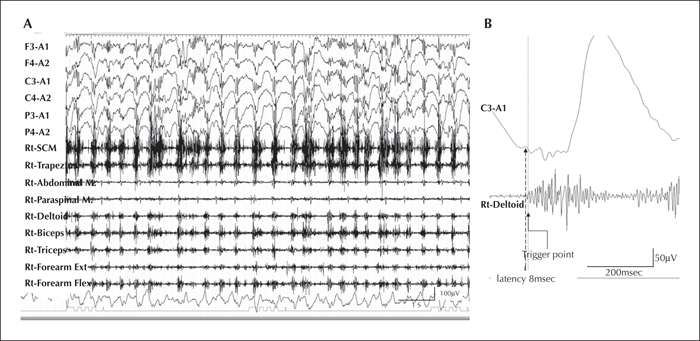
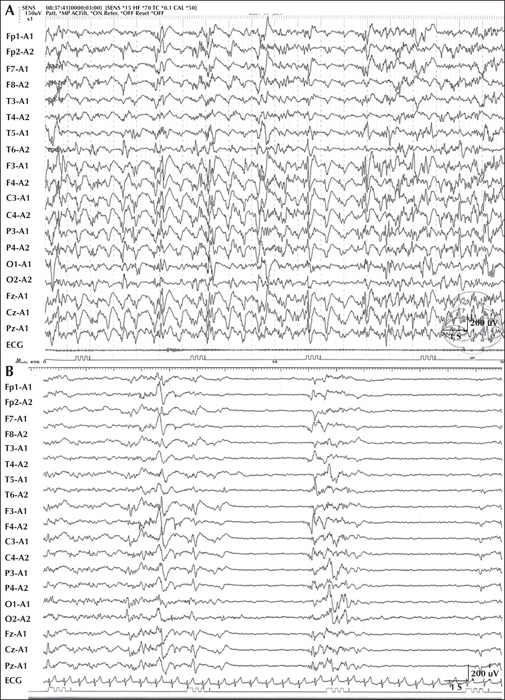
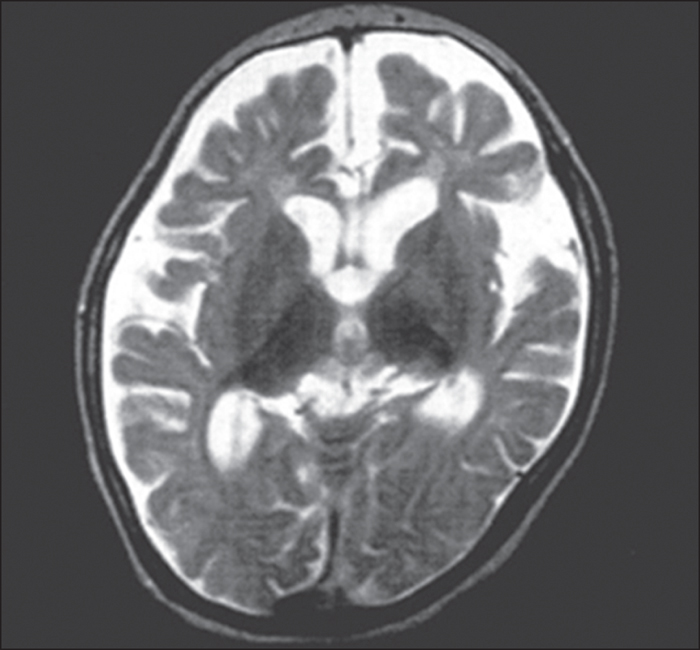
We report a child who developed myoclonic status epilepticus (MSE) at four months of age, associated with rhythmic high-amplitude delta and superimposed (poly) spikes (RHADS), harbouring a GABRB2 (β2 subunit of the GABA A receptor) variant. The patient was treated under a presumptive diagnosis of neonatal-onset Alpers syndrome (AS) and underwent targeted sequence analysis for POLG1 (polymerase gamma 1) and subsequent whole-exome sequence analysis (WES). The patient is currently a 10-year, eight-month-old boy, suffering from daily MSE associated with RHADS and severe global developmental delay from early infancy. Although POLG1 mutation was negative, WES revealed a de novo missense variant of GABRB2 (NM_021911.2: c.784G>T, p.[Val262Phe]). Based on a review of case series with GABRB2 variants, we found that five of the 18 cases shared the clinical and EEG characteristics associated with our patient. In summary, this de novo GABRB2 variant was associated with an AS phenotype, characterized by treatment-resistant MSE and RHADS, and may represent an alternative aetiology for neonatal-onset AS without POLG1 mutation [Published with video sequence].