European Journal of Dermatology
MENUTolerance of bradykinin-releasing drugs in patients with acquired C1 inhibitor deficiency: a case series and review of the literature Volume 32, issue 1, January-February 2022
Authors
1 CHU Lille, Département de Médecine Interne et Immunologie Clinique, F-59000 Lille, France
2 Centre de Référence des Angioedèmes à Kinines, F-59000 Lille, France
3 CHU Lille, Département des Maladies du Sang, F-59000 Lille, France
4 University Lille, U1286 - INFINITE - Institute for Translational Research in Inflammation, F-59000 Lille, France
5 Inserm, F-59000 Lille, France
6 CHU Lille, Département de Dermatologie, F-59000 Lille, France
7 University Lille, Inserm, CHU Lille, UMR-S1172, Center for Pharmacovigilance, F-59000 Lille, France
* Reprints
- Key words: ACE inhibitor, angiotensin receptor blocker, bradykininmediated angioedema, C1-inhibitor deficiency, acquired angioedema
- DOI : 10.1684/ejd.2021.4175
- Page(s) : 49-55
- Published in: 2022
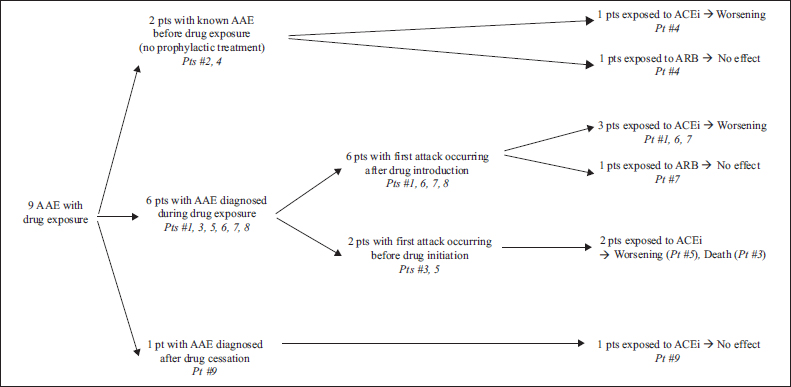
Bradykinin-mediated angioedema (AE) manifests as recurrent oedema of the mucosa, skin and abdominal wall, usually lasting 48 to 96 hours. It can be caused by bradykinin-releasing drugs (such as angiotensin converting enzyme inhibitors [ACEi], angiotensin receptor blockers [ARB] and dipeptidyl peptidase 4 inhibitors [DPP-4i]) or by C1-inhibitor (C1-INH) deficiency, which can be hereditary (HAE) or more rarely acquired (AAE). AAE is classically associated with lymphoid malignancies or monoclonal gammopathy [...]