L'Information Psychiatrique
MENUNon-marketing authorization of (off-label) prescriptions in child psychiatry Volume 94, issue 2, Février 2018
- Key words: medical prescription, child psychiatry, medication, marketing authorization, child, recommendation, regulation
- DOI : 10.1684/ipe.2018.1752
- Page(s) : 101-7
- Published in: 2018
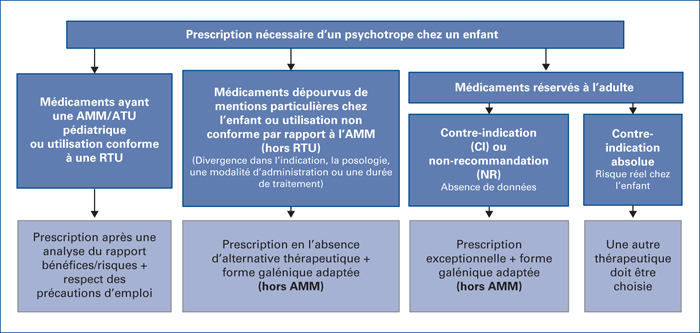
Although child psychiatric care is essentially focused on psychotherapy, sometimes the psychiatrist has to resort to the prescription of one or more drugs. Due to the lack of pharmaceutical specialties with a Paediatric Use Marketing Authorization (AMM) and the limited number of clinical trials in children undertaken, the prescriber is regularly confronted with the practice of using “off-label” drugs, which is full of responsibility problems (i.e. legal risk) and care for the patient (non-reimbursement). Then, after having justified their choice in an argumentative way, the child psychiatrist must respect rules in order to ensure their patients optimal therapeutic management.
![]() This work is licensed under a
Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License
This work is licensed under a
Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License